
Concept explainers
(a)
Interpretation: The conformation which is present in higher concentration when
Concept introduction: The change in Gibbs free energy is represented by
If the
Answer to Problem 6.40P
The equatorial conformation is present in higher concentration in the given compound.
Explanation of Solution
Given
The value of
The equilibrium reaction of monosubstituted cyclohexane is shown below.
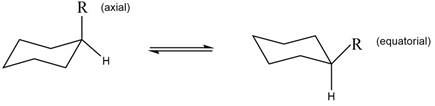
Figure 1
The value of
The equatorial conformation is present in higher concentration in the given compound.
(b)
Interpretation: The
Concept introduction: The change in Gibbs free energy is represented by
If the
Answer to Problem 6.40P
The
Explanation of Solution
The values of
The both given values are greater than
If the
Therefore, the
The
(c)
Interpretation: The
Concept introduction: The change in Gibbs free energy is represented by
If the
Answer to Problem 6.40P
The
Explanation of Solution
The values of
The equilibrium constant
Therefore, the
The
(d)
Interpretation: The
Concept introduction: The change in Gibbs free energy is represented by
If the
Answer to Problem 6.40P
The value of
Explanation of Solution
Given
The values of
The relationship between
As the value of
The value of
(e)
Interpretation: The explanation corresponding to the relation of size of
Concept introduction: The change in Gibbs free energy is represented by
If the
Answer to Problem 6.40P
The large size of
Explanation of Solution
The value
The large size of
Want to see more full solutions like this?
Chapter 6 Solutions
ORG.CHEMISTRY W/ACCESS+MODEL KIT PKG
- Draw a chiral alkene with the formula C6H12.arrow_forwardWhat hydrocarbon with the molecular formula C4H10 forms three monochlorinated products? One is achiral and two are chiral.arrow_forwardDraw the most stable conformation of pentane, using wedges and dashes to represent bonds coming out of the paper and going behind the paper, respectively.arrow_forward
- Draw and name the five cycloalkane structures of formula C5H10. Can any of these structures give rise to geometric (cis-trans) isomerism? If so, show the cis and trans stereoisomersarrow_forwardName the isomers of the compound with the closed formula C4H9Cl. Which of these isomers can have a chiral structure? Examine it from a stereochemical point of view.arrow_forward1. a. Draw and name the five cycloalkane structures of formula C5H10. Can any of these structures give rise to geometric (cis-trans) isomerism? If so, show the cis and trans stereoisomers. b. Draw and name the eight cycloalkane structures of formula C6H12 that do not show geometric isomerism. c. Draw and name the four cycloalkanes of formula C6H12 that do have cis-trans isomers. 2. Each of the following descriptions applies to more than one alkane. In each case, draw and name two structures that match the description. (a) an isopropylheptane (b) a diethyldecane (c) a cis-diethylcyclohexane (d) a trans-dihalocyclopentane (e) a (2,3-dimethylpentyl)cycloalkane (f) a bicyclononane 3. 2. refer to the photo attached and answer the ff.3-33, 3-34arrow_forward
- Consider the following reactions: When C5H12 is reacted with Cl2(g) in the presence of ultraviolet light, four different monochlorination products form. What is the structure of C5H12 in this reaction? When C4H8 is reacted with H2O, a tertiary alcohol is produced as the major product. What is the structure of C4H8 in this reaction? When C7H12 is reacted with HCl, 1-chloro-1-methylcyclohexane is produced as the major product. What are the two possible structures for C7H12 in this reaction? When a hydrocarbon is reacted with water and the major product of this reaction is then oxidized, acetone (2-propanone) is produced. What is the structure of the hydrocarbon in this reaction? When C5H12O is oxidized, a carboxylic acid is produced. What are the possible structures for C5H12O in this reaction?arrow_forwardThe sex attractant of the female tiger moth is an alkane of molecular formula C 18H 38. Is this molecule an acyclic alkane or a cycloalkane?arrow_forwardExplain the concept of Cyclohexane Conformations ?arrow_forward
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning





