
Concept explainers
(a)
Interpretation: Carbocation can rearrange to form a stable carbocation. The carbocation rearrangement with a curve arrow is to be interpreted for the given carbocation.
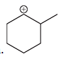
Concept introduction: Carbocation is an intermediate that carries a positive charge on the carbon atom. It tends to rearrange to form a more stable carbocation. The stability order of carbocation is:
Tertiary >Secondary >Primary
The notation to carbocation is given on the basis of the positive charge on the primary, secondary, and tertiary carbon atoms of the intermediate.
(b)
Interpretation: A carbocation can rearrange to form a stable carbocation. The carbocation rearrangement with a curve arrow is to be interpreted for the given carbocation.
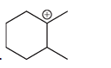
Concept introduction: A carbocation is an intermediate that carries a positive charge on the carbon atom. It tends to rearrange to form a more stable carbocation. The stability order of carbocation is:
Tertiary >Secondary >Primary
The notation to carbocation is given on the basis of the positive charge on the primary, secondary, and tertiary carbon atoms of the intermediate.
(c)
Interpretation: Carbocation can rearrange to form a stable carbocation. The carbocation rearrangement with a curve arrow is to be interpreted for the given carbocation.
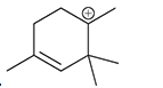
Concept introduction: Carbocation is an intermediate that carries a positive charge on the carbon atom. It tends to rearrange to form a more stable carbocation. The stability order of carbocation is:
Tertiary >Secondary >Primary
The notation to carbocation is given on the basis of the positive charge on the primary, secondary, and tertiary carbon atoms of the intermediate.
(d)
Interpretation: Carbocation can rearrange to form a stable carbocation. The carbocation rearrangement with a curve arrow is to be interpreted for the given carbocation.
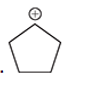
Concept introduction: Carbocation is an intermediate that carries a positive charge on the carbon atom. It tends to rearrange to form a more stable carbocation. The stability order of carbocation is:
Tertiary >Secondary >Primary
The notation to carbocation is given based on the positive charge on the primary, secondary, and tertiary carbon atoms of the intermediate.
(e)
Interpretation: Carbocation can rearrange to form a stable carbocation. The carbocation rearrangement with a curve arrow is to be interpreted for the given carbocation.

Concept introduction: Carbocation is an intermediate that carries a positive charge on the carbon atom. It tends to rearrange to form a more stable carbocation. The stability order of carbocation is:
Tertiary >Secondary >Primary
The notation to carbocation is given on the basis of the positive charge on the primary, secondary, and tertiary carbon atoms of the intermediate.
(f)
Interpretation: Carbocation can rearrange to form a stable carbocation. The carbocation rearrangement with a curve arrow is to be interpreted for the given carbocation.
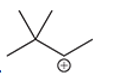
Concept introduction: Carbocation is an intermediate that carries a positive charge on the carbon atom. It tends to rearrange to form more stable carbocation. The stability order of carbocation is:
Tertiary >Secondary >Primary
The notation to carbocation is given on the basis of the positive charge on primary, secondary, and tertiary carbon atoms of the intermediate.
(g)
Interpretation: Carbocation can rearrange to form a stable carbocation. The carbocation rearrangement with a curve arrow is to be interpreted for the given carbocation.
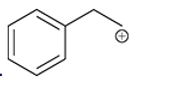
Concept introduction: A carbocation is an intermediate that carries a positive charge on the carbon atom. It tends to rearrange to form more stable carbocation. The stability order of carbocation is:
Tertiary >Secondary >Primary
The notation to carbocation is given on the basis of the positive charge on primary, secondary, and tertiary carbon atoms of the intermediate.
(h)
Interpretation: Carbocation can rearrange to form a stable carbocation. The carbocation rearrangement with a curve arrow is to be interpreted for the given carbocation.
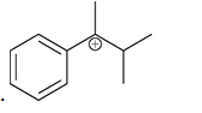
Concept introduction: Carbocation is an intermediate that carries a positive charge on the carbon atom. It tends to rearrange to form a more stable carbocation. The stability order of carbocation is:
Tertiary >Secondary >Primary
The notation to carbocation is given on the basis of the positive charge on primary, secondary and tertiary carbon atom of the intermediate.
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
ORGANIC CHEMISTRY,SOLNS...-ETEXT+BOX
- Each image is part of the question. Need help with part 1 and 2.arrow_forwardEdit the reaction by drawing all steps in the appropriate boxes and connecting them with reaction arrows. Add charges where needed. Electron flow arrows should start on an atom or a bond and should end on an atom, bond, or location where a new bond should be created.arrow_forwardAlkene bromination. The two butane isomers react with Br2. Draw the key reaction inter- mediates and the product(s); use arrow pushing to explain the stereochemical outcomes of the reactions Final Products Intermediate Br2 Intermediate Final Products Br2arrow_forward
- Payalbenarrow_forwardDraw the most stable resonance form for the intermediate in the following electrophilic substitution reaction. LOCH3 LOCH3 HNO3 / CH3CO₂H O₂N • You do not have to consider stereochemistry. • Include all valence lone pairs in your answer. ● In cases where there is more than one answer, just draw one.arrow_forwardDraw the most stable resonance form for the intermediate in the following electrophilic substitution reaction. CI CI HNO3 / H2SO4 O2N You do not have to consider stereochemistry. • Include all valence lone pairs in your answer. • In cases where there is more than one answer, just draw one.arrow_forward
- Draw both resonance structures of the most stable carbocation intermediate in the reaction shown. + HCI •You do not have to consider stereochemistry. Do not include anionic counter-ions, e.g., I, in your answer. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom Separate resonance structures using the → symbol from the drop-down menu. • Draw both resonance structures of the most stable carbocation intermediate in the reaction shown. + HCI • You do not have to consider stereochemistry. • Do not include anionic counter-ions, e.g., I, in your answer. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom righ Separate resonance structures using the symbol from the drop-down menu. • - Draw both resonance structures of the most stable carbocation intermediate in the reaction shown. + HBr • You do not have to consider stereochemistry. Do not include anionic counter-ions, e.g., I, in your answer. •…arrow_forwardDraw both resonance structures of the most stable carbocation intermediate in the reaction shown. Do not include the halide anion. Let Marvin JS adjust the number of hydrogens automatically as you use the charge tool to specify formal charge. + HCI Marvin JS - Troubleshooting Marvin JS - Compatibilityarrow_forwardDraw the most stable resonance form for the intermediate in the following electrophilic substitution reaction. CH3 CH3 CH3COCI/ AICI3 H₂C. You do not have to consider stereochemistry. Include all valence lone pairs in your answer. • In cases where there is more than one answer, just draw one.arrow_forward
- Rank the following carbocations in order of decreasing stability, putting the most stable first. II Multiple Choice |> || > II Il >l> II III > T> || III > || > Tarrow_forwardFollow the curved arrows and draw the product of this reaction. Ph • You do not have to consider stereochemistry.arrow_forwardDraw the most stable resonance form for the intermediate in the following electrophilic substitution reaction. OH CCI3 H2SO4 CCI3 CI- First stage in synthesis of the insecticide DDT, which is now banned in the US You do not have to consider stereochemistry. Include all valence lone pairs in your answer. • In cases where there is more than one answer, just draw one.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

