
ORGANIC CHEMISTRY,SOLNS...-ETEXT+BOX
4th Edition
ISBN: 9781119760634
Author: Klein
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Question
Chapter 6.9, Problem 14ATS
Interpretation Introduction
Interpretation: The arrow pushing pattern for the given mechanism is to be interpreted for the conversion of compound 1 to 2.
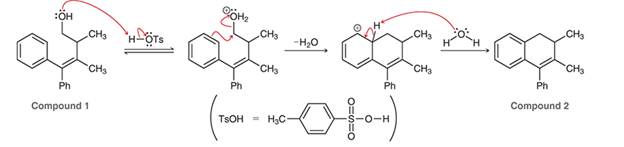
Concept introduction: In a
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
The compound below was exposed to a palladium catalyst to produce a macrocycle (large ring). The macrocycle subsequently
underwent a rearrangement to produce a fused hexacyclic (six-ring) structure. The ring system of the final product is one that is found
in a number of polycyclic natural products. Draw the initial product of the palladium-catalyzed reaction, the final product, and a
mechanism consistent with the rearrangement step.
Include stereochemistry in your answer.
SnBu,
TIO
Integrated Problem 23.67a
* Your answer is incorrect.
Draw the initial product of the palladium-catalyzed reaction:
Synthesize the product from the given material. Give the reagents necessary and draw out any intermediate products along the way.
Under acid-catalyzed conditions, epoxides can be opened by a variety of nucleophiles other than water, such as alcohols. In such a
case, the nucleophile will generally attack at the more substituted position. Using this information, predict the products for each
of the following reactions (a-b):
?
1) RCO-H
2) [H₂SOJ
Modify the given structure to draw the major product(s). Use the single bond tool to interconvert between double and single
bonds. If a racemic mixture of enantiomers is expected, draw both enantiomers. Note: you can select a structure and use Copy and
Paste to save drawing time.
CH₂
Edit Drawing
A
Chapter 6 Solutions
ORGANIC CHEMISTRY,SOLNS...-ETEXT+BOX
Ch. 6.1 - Prob. 1LTSCh. 6.1 - Prob. 1PTSCh. 6.1 - Prob. 2ATSCh. 6.2 - Prob. 3CCCh. 6.3 - Prob. 4CCCh. 6.3 - Prob. 5CCCh. 6.4 - Prob. 6CCCh. 6.6 - Prob. 7CCCh. 6.7 - Prob. 2LTSCh. 6.7 - Prob. 8PTS
Ch. 6.7 - Prob. 9PTSCh. 6.7 - Prob. 10ATSCh. 6.8 - Prob. 3LTSCh. 6.8 - Prob. 11PTSCh. 6.8 - Prob. 12ATSCh. 6.9 - Prob. 4LTSCh. 6.9 - Prob. 13PTSCh. 6.9 - Prob. 14ATSCh. 6.10 - Prob. 5LTSCh. 6.10 - Prob. 15PTSCh. 6.10 - Prob. 16ATSCh. 6.11 - Prob. 6LTSCh. 6.11 - Prob. 17PTSCh. 6.11 - Prob. 18ATSCh. 6 - Prob. 19PPCh. 6 - Prob. 20PPCh. 6 - Prob. 21PPCh. 6 - Prob. 22PPCh. 6 - Prob. 24PPCh. 6 - Prob. 25PPCh. 6 - Prob. 26PPCh. 6 - Prob. 27PPCh. 6 - Prob. 28PPCh. 6 - Prob. 29PPCh. 6 - Prob. 30PPCh. 6 - Prob. 31PPCh. 6 - Prob. 32PPCh. 6 - Prob. 33PPCh. 6 - Prob. 34PPCh. 6 - Prob. 35PPCh. 6 - Prob. 36PPCh. 6 - Prob. 37PPCh. 6 - Prob. 38PPCh. 6 - Prob. 39PPCh. 6 - Prob. 40PPCh. 6 - Prob. 41PPCh. 6 - Prob. 43ASPCh. 6 - Prob. 44ASPCh. 6 - Prob. 45ASPCh. 6 - Prob. 46ASPCh. 6 - Prob. 47ASPCh. 6 - Prob. 48ASPCh. 6 - Prob. 49ASPCh. 6 - Prob. 50IPCh. 6 - Prob. 51IPCh. 6 - Prob. 52IPCh. 6 - Prob. 53IPCh. 6 - Prob. 54IPCh. 6 - Prob. 55IPCh. 6 - Prob. 56IPCh. 6 - Prob. 57IPCh. 6 - Prob. 58IPCh. 6 - Prob. 59IPCh. 6 - Prob. 60IPCh. 6 - Prob. 61IPCh. 6 - Prob. 62CPCh. 6 - Prob. 64CP
Knowledge Booster
Similar questions
- A problem often encountered in the oxidation of primary alcohols to acids is that esters are sometimes produced as by-products. For example, oxidation of ethanol yields acetic acid and ethyl acetate: Propose a mechanism to account for the formation of ethyl acetate. Take into account the reversible reaction between aldehydes and alcohols:arrow_forwardThe compound eutypine is an antibacterial agent isolated from the fungus Eutypa lata. This fungus results in a disease common to vineyards called eutyposis. Give a sequence of reactions that will take the following reactant and give eutypine when the other reactants used in the sequence are acetylene and acetone.arrow_forward58) Draw the product resulting from the following reaction. Indicate any relevant stereo chemistry. Eto EtOHarrow_forward
- Acid-catalyzed bromination of pentan-2-one (CH3COCH2CH2CH3) forms two products: BrCH2COCH2CH2CH3 (A)and CH3COCH(Br)CH2CH3 (B). Explain why the major product is B, with the Br atom on the more substituted side of the carbonyl group.arrow_forwardThe high reactivity of alkyl halides can be explained in terms of nature of C-X bond which is highly polarized covalent bond due to large difference in the electronegativities of carbon and halogen atom. This polarity is responsible for the nucleophilic substitution reactions of alkyl halides which mostly occur by Swa and Swa mechanisms. Sy reaction is a two-step process and in the first step, R-X ionizes to give carbocation (slow process). In the second step, the nucleophile attacks the carbocation from either side to form the product (fast process). In Swi reaction, there can be racemization and inversion. Swi reaction is favored by heavy (bulky) groups on the carbon atom attached to halogens. i.e., R,C-X>R;CH-X>R-CH,X>CH,X. In Sna reaction, the strong nucleophile OH attacks from the opposite side of the chlorine atom to give an intermediate (transition state) which breaks to yield the product (alcohol) and leaving (X) group. The alcohol has a configuration opposite to that of the…arrow_forwardWhich of the following undergoes a substitution reaction with sodium cyanide in DMSO at the fastest rate? a) CH3CH2F b) CH3CH2Cl c) CH3CH2Br d) CH3CH2Iarrow_forward
- Secondary alcohols are often dehydrated in an E2 reaction to give an alkene. Elimination follows Zaitsev's rule to give the more substituted alkene as the major product. Since the reaction occurs via an E2 mechanism, there is no risk of rearrangement of the carbon skeleton as could possibly occur if the elimination occurred via an E1 mechanism with a carbocation intermediate.Draw curved arrows to show the movement of electrons in this step of the mechanism.arrow_forwardFluorination of a benzene ring can be accomplished with Selectfluor, a reagent that contains a fluorine bonded to a positively charged nitrogen atom. Fluorination is a useful reaction because several common drugs, such as the cholesterol-lowering drug atorvastatin, contain a fluorine bonded to an aromatic ring. Assuming that fluorination is analogous to other examples of electrophilic aromatic substitution, draw a stepwise mechanism for the following reaction.arrow_forwardFluorination of a benzene ring can be accomplished with Selectfluor, a reagent that contains a fluorine bonded to a positively charged nitrogen atom. Fluorination is a useful reaction because several common drugs, such as the cholesterol-lowering drug atorvastatin, contain a fluorine bonded to an aromatic ring. Assuming that fluorination is analogous to other examples of electrophilic aromatic substitution, draw a stepwise mechanism for the following reaction.arrow_forward
- Terreic acid, shown below, is a naturally occurring antibiotic metabolite of the fungus Aspergillus terreus. Terreic acid hinders bacterial growth by covalently binding to (and thereby deactivating) the biosynthetic enzyme MurA, which is responsible for synthesizing the bacterial cell wall. In aqueous environments, terreic acid tautomerizes to a more stable enol form. Draw the two most stable enol tautomers of terric acid. Circle the tautomer above that you would expect to predominate inside a cellular environment.arrow_forwardShow how to convert toluene to 3-hydroxybenzoic acid using the same set of reactions as in diagram but changing the order in which two or more of the steps are carried outarrow_forward(c) Draw the chemical structure of the main product (10) obtained in reaction [6] and provide a detailed mechanism for its formation from the most stable conformation of the starting compound. CI NaOEt, heat (10) [6] ****Oarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
