
Concept explainers
Each gram of mammalian skeletal muscle consumes ATP at a rate of about 1 × 10-3 mol/min during contraction. To bridge the short interval between the moderate demand for ATP met by aerobic
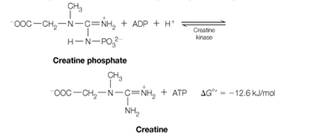
Because the equilibrium lies well to the right, virtually all of the muscle ADP or AMP is converted to ATP as long as creatine phosphate is available. Concentrations of ATP and creatine phosphate in muscle are about 4 mM and 25 mM, respectively, and the density of muscle tissue can be taken to be about 1.2 g/ cm3.
a. How long could contraction continue using ATP alone?
b. If all creatine phosphate were converted into ATP and utilized as well, how long could contraction continue?
c. What do these answers tell you about the role of ATP in providing energy to cells?
Want to see the full answer?
Check out a sample textbook solution
Chapter 7 Solutions
Modified Masteringchemistry With Pearson Etext -- Format: Access Card Package
- Assuming that all the glucose entering a muscle fiber is oxidized, as what molecule will the glucose carbon leave the body? Of glucose’s six carbons, two oxidized carbon byproducts will be produced by one enzyme, and four will be produced by a metabolic pathway. At which enzyme and pathway are these carbon-based bi-products produced?arrow_forwardIntramitochondrial ATP concentrations are about 5 mM, and phosphate concentration is about 10 mM. If ADP is five times more abundant than AMP,calculate the molar concentrations of ADP and AMP at an energy charge of 0.85. Calculate ∆G for ATP hydrolysis at 37 °C under these conditions.The energy charge is the concentration of ATP plus half the concentration of ADP divided by the total adenine nucleotide concentration.arrow_forwardHow many ATPs are synthesized for every cytoplasmic NADH that participates in the glycerophosphate shuttle in insect flight muscle?arrow_forward
- When you hold a weight at arm's length, you are not doing any thermodynamic work but the muscles supporting the weight are nevertheless consuming energy. Describe, on the molecular level, how muscles might maintain such state of constant tension without contracting. Why does this state consume ATP?arrow_forwardis this stement false? Intracellular concentrations in resting muscle are as follows: Fructose-6-phosphate (1.0 mM)Fructose-(1-6)-bisphosphate (10.0 mM)AMP (0.1 mM)ADP (0.5 mM)ATP (5.0 mM)Pi (10.0 mM)Under the above conditions the Phosphofructokinase reaction in muscle is more exergonic than under standard conditions.arrow_forwardHow many protons are required to synthesize one ATP by F1F0-ATPase containing (a) 10 or (b) 15 c subunits?arrow_forward
- Calcium is an important regulator of the citric acid cycle. Calcium is transported across the mitochondrial inner membrane by a Ca²- 2+ uniporter that is driven by the negative potential inside the matrix. Part A Assuming a membrane potential across the inner membrane of 172 mV (inside negative), calculate the ratio of the [Ca2+] in the matrix to that in the cytoplasm ([Ca2+]m/[Ca²+]c) that would exist at equilibrium (i.e., AG = 0). Express your answer using two significant figures. [Ca2+]m [Ca2+] = ΤΟ ΑΣΦ Submit Request Answer Part B ? Cytoplasmic [Ca2+] is on the order of 10-7 M in a healthy cell. Based on your answer in Part A, calculate the [Ca2+] that would exist in the matrix at equilibrium. Express your answer to two significant figures and include the appropriate units. ☐ μÅ ? Value Unitsarrow_forwardIn angiogenic endothelial cells, pyruvate is converted to lactate (generating 2 ATP per glucose) rather than being completely oxidized (which would generate ~32 ATP by oxidative phosphorylation). Explain why angiogenic cells generate ATP anaerobically.arrow_forwardRat heart muscle operating aerobically fills more than 90% of its ATP needs by oxidative phosphorylation. If each gram of tissue consumes O2 at the rate of 12.0 micromol/min, with glucose as the fuel source. (a) Calculate the rate at which the heart muscle consumes glucose and produces ATP. (b) Consider an alternate scenario – what would be the rate of consumption if the energy source was a solely triglycerides whose fatty acyl chains were each 14-C in length and saturated? (Assume the O2 consumption rate remains at 12.0 micromol/min) (c) For a steady-state concentration of ATP of 6.0 micromol/g of heart muscle tissue, calculate the time required (in seconds) to completely turn over the cellular pool of ATP if glucose us used as the sole fuel source. What does this result indicate about the need for tight regulation of ATP production? (Note: Concentrations are expressed as micromoles per gram of muscle tissue because the tissue is mostly water.)arrow_forward
- A critical reaction in the production of energy to do work or drive chemical reactions in biological systems is the hydrolysis of adenosine triphosphate, ATP, to adenosine diphosphate, ADP, as described by the reaction ATP(aq)+H2O(l)⟶ADP(aq)+HPO2−4(aq)ATP(aq)+H2O(l)⟶ADP(aq)+HPO42−(aq) for which Δ?∘rxn=−30.5 kJ/molΔGrxn∘=−30.5 kJ/mol at 37.0 °C and pH 7.0. Calculate the value of Δ?rxnΔGrxn in a biological cell in which [ATP]=5.0 mM,[ATP]=5.0 mM, [ADP]=0.10 mM,[ADP]=0.10 mM, and [HPO2−4]=5.0 mM.[HPO42−]=5.0 mM. Δ?rxn=ΔGrxn= kJ/molarrow_forwardUnlike a rabbit, running all-out for a few moments to escape a predator, migratory birds require energy for extended periods of time. For example, ducks generally fly several thousand miles during their annual migration. The flight muscles of migratory birds have a high oxidative capacity and obtain the necessary ATP through the oxidation of acetyl-CoA (obtained from fats) via the citric acid cycle. Compare the regulation of muscle glycolysis during short-term intense activity, as in a fleeing rabbit, and during extended activity, as in the migrating duck. Why must the regulation in these two settings be different? Extended activity requires the highly efficient anaerobic metabolism of fats, rather than the less efficient aerobic metabolism of glucose. Extended activity requires the highly efficient aerobic oxidation of fats, rather than the less efficient anaerobic metabolism of glucose. Extended activity stimulates glycolysis because the concentrations of citrate and acetyl-CoA are…arrow_forwardIn active muscle cells, the pO2 is about 10 torr at the cell surface and 1 torr at the mitochondria(the organelles where oxidative metabolism occurs). Calculate the percentage of bound oxygentransported to the mitochondria of muscle cells by myoglobin (KD = 2 torr).arrow_forward
 BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Human Physiology: From Cells to Systems (MindTap ...BiologyISBN:9781285866932Author:Lauralee SherwoodPublisher:Cengage Learning
Human Physiology: From Cells to Systems (MindTap ...BiologyISBN:9781285866932Author:Lauralee SherwoodPublisher:Cengage Learning

