
World of Chemistry
7th Edition
ISBN: 9780618562763
Author: Steven S. Zumdahl
Publisher: Houghton Mifflin College Div
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 7, Problem 48A
Interpretation Introduction
Interpretation: A balanced chemical equation for the given process needs to be determined.
Concept Introduction: When one or more chemicals are changed into one or more other chemicals, a
Expert Solution & Answer
Answer to Problem 48A
Barium chloride (BaCl2) reacts with potassium chromate (K2Cr2O7) in aqueous solution-
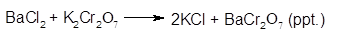
Explanation of Solution
The Barium chromate is low solubility in cold water at a concentration and thus yellow precipitate will form.
The balanced chemical equation for the reaction will be:
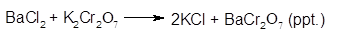
Chapter 7 Solutions
World of Chemistry
Ch. 7.1 - Prob. 1RQCh. 7.1 - Prob. 2RQCh. 7.1 - Prob. 3RQCh. 7.2 - Prob. 1RQCh. 7.2 - Prob. 2RQCh. 7.2 - Prob. 3RQCh. 7.2 - Prob. 4RQCh. 7.3 - Prob. 1RQCh. 7.3 - Prob. 2RQCh. 7.3 - Prob. 3RQ
Ch. 7.3 - Prob. 4RQCh. 7.3 - Prob. 5RQCh. 7 - Prob. 1ACh. 7 - Prob. 2ACh. 7 - Prob. 3ACh. 7 - Prob. 4ACh. 7 - Prob. 5ACh. 7 - Prob. 6ACh. 7 - Prob. 7ACh. 7 - Prob. 8ACh. 7 - Prob. 9ACh. 7 - Prob. 10ACh. 7 - Prob. 11ACh. 7 - Prob. 12ACh. 7 - Prob. 13ACh. 7 - Prob. 14ACh. 7 - Prob. 15ACh. 7 - Prob. 16ACh. 7 - Prob. 17ACh. 7 - Prob. 18ACh. 7 - Prob. 19ACh. 7 - Prob. 20ACh. 7 - Prob. 21ACh. 7 - Prob. 22ACh. 7 - Prob. 23ACh. 7 - Prob. 24ACh. 7 - Prob. 25ACh. 7 - Prob. 26ACh. 7 - Prob. 27ACh. 7 - Prob. 28ACh. 7 - Prob. 29ACh. 7 - Prob. 30ACh. 7 - Prob. 31ACh. 7 - Prob. 32ACh. 7 - Prob. 33ACh. 7 - Prob. 34ACh. 7 - Prob. 35ACh. 7 - Prob. 36ACh. 7 - Prob. 37ACh. 7 - Prob. 38ACh. 7 - Prob. 39ACh. 7 - Prob. 40ACh. 7 - Prob. 41ACh. 7 - Prob. 42ACh. 7 - Prob. 43ACh. 7 - Prob. 44ACh. 7 - Prob. 45ACh. 7 - Prob. 46ACh. 7 - Prob. 47ACh. 7 - Prob. 48ACh. 7 - Prob. 49ACh. 7 - Prob. 1STPCh. 7 - Prob. 2STPCh. 7 - Prob. 3STPCh. 7 - Prob. 4STPCh. 7 - Prob. 5STPCh. 7 - Prob. 6STPCh. 7 - Prob. 7STPCh. 7 - Prob. 8STPCh. 7 - Prob. 9STPCh. 7 - Prob. 10STPCh. 7 - Prob. 11STPCh. 7 - Prob. 12STP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Types of Matter: Elements, Compounds and Mixtures; Author: Professor Dave Explains;https://www.youtube.com/watch?v=dggHWvFJ8Xs;License: Standard YouTube License, CC-BY