
(a)
Interpretation:
To draw the given structure corresponding to each name.
Concept introduction:
Answer to Problem 7.45P
Given the structure name is isopropyl bromide
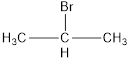
Explanation of Solution
To write the corresponding structure. To work backward from a name to the structure.
First find the parent name and draw it according to the number of carbons. Use the suffix to identify the functional group.
Then arrange the number of carbon in the chain. Finally add the substituent to the appropriate carbon.
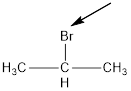
The bromide is in the middle carbon make it into a isopropyl.
Thus given the structure name is isopropyl bromide

(b)
Interpretation:
To draw the given structure corresponding to each name.
Concept introduction:
Alkyl halides are organic molecules that contains a halogen atom X bonded to sp3 hybridized carbon atom. Alkyl halides are classified as primary (1°), secondary (2°), and tertiary (3°) depending on the number of carbons bonded to the carbon with the halogen. An alkyl halide is named as an alkane with a halogen substituent-that is, as a halo alkane. To name a halogen substituent, change the -ine ending of the name of the halogen to the suffix -o (chlorine-chloro).
Answer to Problem 7.45P
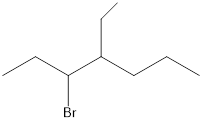
Given the structure name is 3-bromo-4-ethylheptane
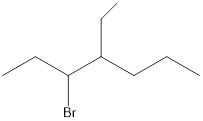
Explanation of Solution
To write the corresponding structure. To work backward from a name to the structure.
First find the parent name and draw it according to the number of carbons. Use the suffix to identify the functional group.
Then arrange the number of carbon in the chain. Finally add the substituent to the appropriate carbon.
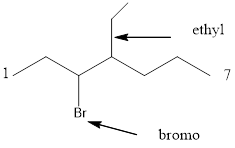
Starting from first carbon the bromide is in the third carbon and ethyl is in fourth carbon, and there are total of seven carbon so 3-bromo-4-ethylheptane.
Thus given the structure name is 3-bromo-4-ethylheptane
(c)
Interpretation:
To draw the given structure corresponding to each name.
Concept introduction:
Alkyl halides are organic molecules that contains a halogen atom X bonded to sp3 hybridized carbon atom. Alkyl halides are classified as primary (1°), secondary (2°), and tertiary (3°) depending on the number of carbons bonded to the carbon with the halogen. An alkyl halide is named as an alkane with a halogen substituent-that is, as a halo alkane. To name a halogen substituent, change the -ine ending of the name of the halogen to the suffix -o (chlorine-chloro).
Answer to Problem 7.45P
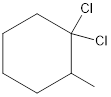
Given the structure name is 1, 1-dichloro-2-methylcyclohexane
Explanation of Solution
To write the corresponding structure. To work backward from a name to the structure.
First find the parent name and draw it according to the number of carbons. Use the suffix to identify the functional group.
Then arrange the number of carbon in the chain. Finally add the substituent to the appropriate carbon.
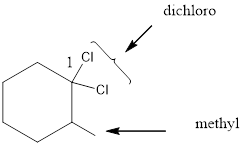
Starting from first carbon the two chlorine is in the first carbon and methyl is in second carbon, and there are total of six carbon and ring so 1, 1-dichloro-2-methylcyclohexane.
Thus Given the structure name is 1, 1-dichloro-2-methylcyclohexane

(d)
Interpretation:
To draw the given structure corresponding to each name.
Concept introduction:
Alkyl halides are organic molecules that contains a halogen atom X bonded to sp3 hybridized carbon atom. Alkyl halides are classified as primary (1°), secondary (2°), and tertiary (3°) depending on the number of carbons bonded to the carbon with the halogen. An alkyl halide is named as an alkane with a halogen substituent-that is, as a halo alkane. To name a halogen substituent, change the -ine ending of the name of the halogen to the suffix -o (chlorine-chloro).
Answer to Problem 7.45P
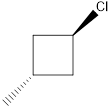
Given the structure name is trans-1-chloro-3-iodocyclobutane
Explanation of Solution
To write the corresponding structure. To work backward from a name to the structure.
First find the parent name and draw it according to the number of carbons. Use the suffix to identify the functional group.
Then arrange the number of carbon in the chain. Finally add the substituent to the appropriate carbon.
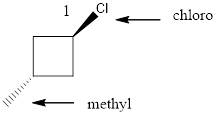
Starting from first carbon the chlorine is in the first carbon and methyl is in third carbon, and there are total of four carbon and ring so trans-1-chloro-3-iodocyclobutane.
Thus Given the structure name is trans-1-chloro-3-iodocyclobutane

(e)
Interpretation:
To draw the given structure corresponding to each name.
Concept introduction:
Alkyl halides are organic molecules that contains a halogen atom X bonded to sp3 hybridized carbon atom. Alkyl halides are classified as primary (1°), secondary (2°), and tertiary (3°) depending on the number of carbons bonded to the carbon with the halogen. An alkyl halide is named as an alkane with a halogen substituent-that is, as a halo alkane. To name a halogen substituent, change the -ine ending of the name of the halogen to the suffix -o (chlorine-chloro).
Answer to Problem 7.45P
Given the structure name is 1-bromo-4-ethyl-3-fluorooctane
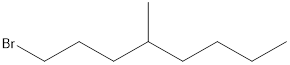
Explanation of Solution
To write the corresponding structure. To work backward from a name to the structure.
First find the parent name and draw it according to the number of carbons. Use the suffix to identify the functional group.
Then arrange the number of carbon in the chain. Finally add the substituent to the appropriate carbon.
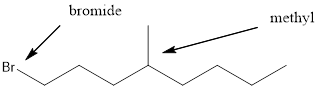
Starting from first carbon the bromide is in the first carbon and methyl is in fourth carbon, and there are total of eight carbon so 1-bromo-4-ethyl-3-fluorooctane.
Thus Given the structure name is 1-bromo-4-ethyl-3-fluorooctane

(f)
Interpretation:
To draw the given structure corresponding to each name.
Concept introduction:
Alkyl halides are organic molecules that contains a halogen atom X bonded to sp3 hybridized carbon atom. Alkyl halides are classified as primary (1°), secondary (2°), and tertiary (3°) depending on the number of carbons bonded to the carbon with the halogen. An alkyl halide is named as an alkane with a halogen substituent-that is, as a halo alkane. To name a halogen substituent, change the -ine ending of the name of the halogen to the suffix -o (chlorine-chloro).
Answer to Problem 7.45P
Given the structure name is (3S)-3-iodo-2-methylnonane
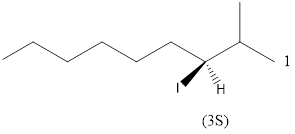
Explanation of Solution
To write the corresponding structure. To work backward from a name to the structure.
First find the parent name and draw it according to the number of carbons. Use the suffix to identify the functional group.
Then arrange the number of carbon in the chain. Finally add the substituent to the appropriate carbon.
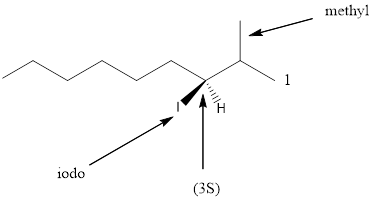
Starting from first carbon the iodo is in the third carbon and methyl is in second carbon, and there are total of nine carbon so (3S)-3-iodo-2-methylnonane.
Thus Given the structure name is (3S)-3-iodo-2-methylnonane

(g)
Interpretation:
To draw the given structure corresponding to each name.
Concept introduction:
Alkyl halides are organic molecules that contains a halogen atom X bonded to sp3 hybridized carbon atom. Alkyl halides are classified as primary (1°), secondary (2°), and tertiary (3°) depending on the number of carbons bonded to the carbon with the halogen. An alkyl halide is named as an alkane with a halogen substituent-that is, as a halo alkane. To name a halogen substituent, change the -ine ending of the name of the halogen to the suffix -o (chlorine-chloro).
Answer to Problem 7.45P
Given the structure name is (1 R, 2R)-trans-1-bromo-2-chlorocyclohexane
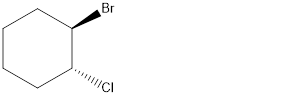
Explanation of Solution
To write the corresponding structure. To work backward from a name to the structure.
First find the parent name and draw it according to the number of carbons. Use the suffix to identify the functional group.
Then arrange the number of carbon in the chain. Finally add the substituent to the appropriate carbon.
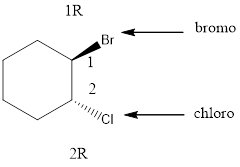
Starting from first carbon the chloro and bromo is in the first carbon and there are total of six carbon so (1 R, 2R)-trans-1-bromo-2-chlorocyclohexane.
Thus Given the structure name is (1 R, 2R)-trans-1-bromo-2-chlorocyclohexane
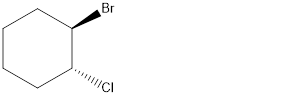
(h)
Interpretation:
To draw the given structure corresponding to each name.
Concept introduction:
Alkyl halides are organic molecules that contains a halogen atom X bonded to sp3 hybridized carbon atom. Alkyl halides are classified as primary (1°), secondary (2°), and tertiary (3°) depending on the number of carbons bonded to the carbon with the halogen. An alkyl halide is named as an alkane with a halogen substituent-that is, as a halo alkane. To name a halogen substituent, change the -ine ending of the name of the halogen to the suffix -o (chlorine-chloro).
Answer to Problem 7.45P
Given the structure name is (5R)-4, 4, 5-trichloro-3, 3-dimethyldecane
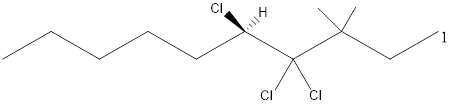
Explanation of Solution
To write the corresponding structure. To work backward from a name to the structure.
First find the parent name and draw it according to the number of carbons. Use the suffix to identify the functional group.
Then arrange the number of carbon in the chain. Finally add the substituent to the appropriate carbon.
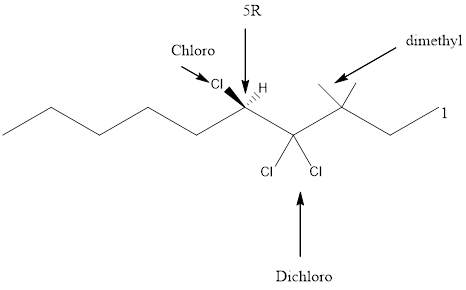
Starting from first carbon the chloro is in the fourth and fifth carbon and there are total of ten carbons so (5R)-4, 4, 5-trichloro-3, 3-dimethyldecane.
Thus Given the structure name is (5R)-4, 4, 5-trichloro-3, 3-dimethyldecane

Want to see more full solutions like this?
Chapter 7 Solutions
ORGANIC CHEMISTRY-STUDY GDE...-W/ACCESS
- KMnO4, warm, conc'd reacts with hept-1-ene to yield __________. CO2, hex-1-ene CO2, hexanoic acid Formic acid, pentanoic acid Ethanoic acid, pentanal Formic acid, hexanonearrow_forwardbest condition for the reaction (1r,2s)-1 bromo-2methylcyclohexane->(s)3-methylcyclohex-1enearrow_forwardDraw out a reaction for 2-chloro-2-methylbutane and KOH, naming each product (if more than one)arrow_forward
- Answer all of question 1 pls 1.i. Give two examples each of Unsymmetrical alkenes and reagents.ii. Give two examples of reactions of alkenes that result in Anti-Markonikov’s addition productsarrow_forwardShow all steps and reagents needed to convert cyclohexane into each compound: (a) the two enantiomers of trans-1,2- dibromocyclohexane; and (b) 1,2-epoxycyclohexane.arrow_forwardOxidation of Butanol -1 in KMnO4/H+ will produce-------------. a.Butanoic Acid b.Butanal c.Pentanoic acid d.Butene -2arrow_forward
- Phineas and Ferbs, two brothers who enjoy vacations, doing fun things every summer. This summer the brothers and their friends carry out an organic synthesis with an unknown compound (L1) that contains 52% Carbon, 6% Hydrogen and 42% bromine, this compound (L1) is treated with magnesium in ether to obtain L2 , which reacts violently with D2O for 1-methyl cyclohexene with a deuterium atom in the methyl group (L3). The L2 reaction is treated with acetone followed by hydrolysis to give L4. Heating L4 with concentrated sulfuric acid gives L5, which decolors the bromine, obtaining L6. L5 undergoes hydrogenation with excess hydrogen and platinum as a catalyst giving rise to isobutyl cyclohexane. Determine the structures of compounds L1 through L6.arrow_forwardHow many products are possible from the reaction ? Take stereochemistry into accountarrow_forwardA hydrocarbon of unknown structure has the formula C8H10. On catalytichydrogenation over the Lindlar catalyst, 1 equivalent of H2 is absorbed. Onhydrogenation over a palladium catalyst, 3 equivalents of H2 are absorbed.(a) How rnany degrees of unsaturation are present in the unknown?(b) How many triple bonds are present?(c) How many double bonds are present?(d) How many rings ar e present?(e) Draw a structure that fits the data.arrow_forward
- A chemist allows some pure (2S,3R)-3-bromo-2,3-diphenylpentane to react with a solution of sodium ethoxide(NaOCH2CH3) in ethanol. The products are two alkenes: A (cis-trans mixture) and B, a single pure isomer. Under thesame conditions, the reaction of (2S,3S)-3-bromo-2,3-diphenylpentane gives two alkenes, A (cis-trans mixture) and C.Upon catalytic hydrogenation, all three of these alkenes (A, B, and C) give 2,3-diphenylpentane. Determine the structuresof A, B, and C; give equations for their formation; and explain the stereospecificity of these reactionsarrow_forwardAssign the stereochemical configuration (E or Z) for the alkene below. Show your work, indicating clearly which groups are assigned high priority (e.g., through assigning the groups numbers, circling only the high priority groups, or labeling groups as high or low).arrow_forwardWhat would the final products look like? Pls specify stereochemistry if neededarrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
