
(a)
Interpretation:
The one which is larger among methyl or phenyl is to be stated.
Concept introduction:
In a cyclohexane ring, the steric interactions that takes place between an axial substituent located on the carbon atom 1 and the hydrogen atoms located on carbon 3 and 5 is known as 1, 3 diaxial interaction. In the cyclohexane derivatives, each 1, 3-diaxial interaction between the methyl and hydrogen increases the enthalpy of the ring by
Answer to Problem 7.73AP
The value of
Explanation of Solution
The two conformations of cyclohexane are given below.
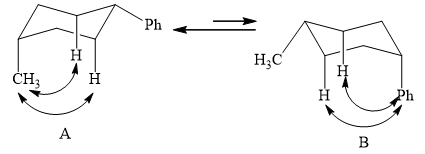
Figure 1
In the above shown figure, in conformation
The phenyl group is more bulky than the methyl group. So, it will have a destabilizing effect, and therefore, the equilibrium is favored towards conformation
The formula to calculate
The energy for every methyl-hydrogen
The
Substitute the values of energy and
The value of
The equilibrium is favored towards compound
(b)
Interpretation:
The value of
Concept introduction:
In a cyclohexane ring, the steric interactions that takes place between an axial substituent located on the carbon atom 1 and the hydrogen atoms located on carbon 3 and 5 is known as 1, 3 diaxial interaction. In the cyclohexane derivatives, each 1, 3-diaxial interaction between the methyl and hydrogen increases the enthalpy of the ring by
Answer to Problem 7.73AP
(1) The value of
(2) The value of
Explanation of Solution
(1) The two conformations of cyclohexane are given below.
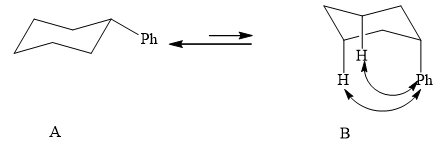
Figure 2
In the above shown figure, in conformation
The formula to calculate
The value of
Substitute the value of energy into the above equation.
(2) The two conformations of cyclohexane are given below.
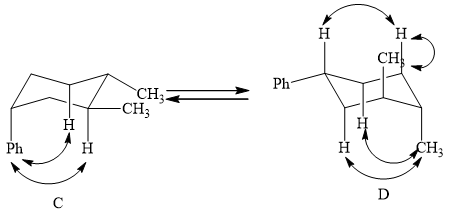
Figure 3
In the above shown figure, in conformation
The formula to calculate
The energy for every methyl-hydrogen
The value of
Substitute the values of energy into the above equation.
The value of
Want to see more full solutions like this?
Chapter 7 Solutions
ORGANIC CHEMISTRY SAPLING ACCESS + ETEX
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





