
EBK GENERAL, ORGANIC, & BIOLOGICAL CHEM
3rd Edition
ISBN: 9781259298424
Author: SMITH
Publisher: VST
expand_more
expand_more
format_list_bulleted
Question
Chapter 7.11, Problem 7.35P
Interpretation Introduction
Interpretation:
Whether the process represented in the molecular art is endothermic or exothermic needs to be determined.
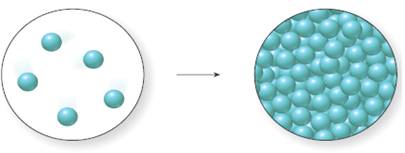
Concept introduction:
The exothermic process is defined as the process in which the energy is liberated during the conversion of one state of substance to another state of substance.
The endothermic process is defined as the process in which the energy is absorbed during the conversion of one state of substance to that of another state of substance.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Classify each process as exothermic or endothermic.
(a) gasoline burning in a car(b) isopropyl alcohol evaporating from skin(c) water condensing as dew during the night
Is the solution of ammonium chloride in water an exothermic or endothermic process.
Is the process shown in the figureendothermic or exothermic?
Chapter 7 Solutions
EBK GENERAL, ORGANIC, & BIOLOGICAL CHEM
Ch. 7.1 - Prob. 7.1PCh. 7.2 - Convert each pressure unit to the indicated unit....Ch. 7.3 - Prob. 7.3PCh. 7.3 - Prob. 7.4PCh. 7.3 - Prob. 7.5PCh. 7.3 - Prob. 7.6PCh. 7.3 - Prob. 7.7PCh. 7.3 - Prob. 7.8PCh. 7.3 - The pressure inside a 1.0-L balloon at 25C was 750...Ch. 7.4 - A sample of nitrogen gas contains 5.0 mol in a...
Ch. 7.4 - Prob. 7.11PCh. 7.4 - Prob. 7.12PCh. 7.5 - Prob. 7.13PCh. 7.5 - Prob. 7.14PCh. 7.6 - CO2 was added to a cylinder containing 2.5 atm of...Ch. 7.6 - Prob. 7.16PCh. 7.6 - Prob. 7.17PCh. 7.7 - Prob. 7.18PCh. 7.7 - Prob. 7.19PCh. 7.7 - Prob. 7.20PCh. 7.7 - Which species in each pair has stronger...Ch. 7.7 - Prob. 7.22PCh. 7.7 - Prob. 7.23PCh. 7.8 - Prob. 7.24PCh. 7.8 - Would you predict the surface tension of gasoline,...Ch. 7.9 - Prob. 7.26PCh. 7.10 - Prob. 7.27PCh. 7.10 - The human body is composed of about 70% water. How...Ch. 7.10 - How much energy is required to heat 28.0 g of iron...Ch. 7.10 - Prob. 7.30PCh. 7.10 - Prob. 7.31PCh. 7.10 - If the initial temperature of 120. g of ethanol is...Ch. 7.11 - Use the heat of fusion of water from Sample...Ch. 7.11 - Answer the following questions about water, which...Ch. 7.11 - Prob. 7.35PCh. 7.12 - Answer the following questions about the graph...Ch. 7.12 - How much energy (in calories) is released when...Ch. 7.12 - How much energy (in calories) is required to melt...Ch. 7 - Prob. 7.39PCh. 7 - Prob. 7.40PCh. 7 - Prob. 7.41PCh. 7 - The compressed air tank of a scuba diver reads...Ch. 7 - Assume that each of the following samples is at...Ch. 7 - Use the diagrams in Problem 7.43 to answer the...Ch. 7 - Prob. 7.45PCh. 7 - Prob. 7.46PCh. 7 - Prob. 7.47PCh. 7 - Prob. 7.48PCh. 7 - Prob. 7.49PCh. 7 - Prob. 7.50PCh. 7 - Prob. 7.51PCh. 7 - Prob. 7.52PCh. 7 - Prob. 7.53PCh. 7 - If someone takes a breath and the lungs expand...Ch. 7 - Prob. 7.55PCh. 7 - Prob. 7.56PCh. 7 - Prob. 7.57PCh. 7 - Prob. 7.58PCh. 7 - Prob. 7.59PCh. 7 - Prob. 7.60PCh. 7 - Prob. 7.61PCh. 7 - Prob. 7.62PCh. 7 - Prob. 7.63PCh. 7 - Prob. 7.64PCh. 7 - Prob. 7.65PCh. 7 - Prob. 7.66PCh. 7 - Prob. 7.67PCh. 7 - Prob. 7.68PCh. 7 - Prob. 7.69PCh. 7 - Prob. 7.70PCh. 7 - Prob. 7.71PCh. 7 - Prob. 7.72PCh. 7 - Prob. 7.73PCh. 7 - Prob. 7.74PCh. 7 - Prob. 7.75PCh. 7 - Prob. 7.76PCh. 7 - Prob. 7.77PCh. 7 - Prob. 7.78PCh. 7 - Prob. 7.79PCh. 7 - Prob. 7.80PCh. 7 - Prob. 7.81PCh. 7 - Prob. 7.82PCh. 7 - Prob. 7.83PCh. 7 - Prob. 7.84PCh. 7 - Which molecules are capable of intermolecular...Ch. 7 - Prob. 7.86PCh. 7 - Prob. 7.87PCh. 7 - Explain why the boiling point of A is higher than...Ch. 7 - Prob. 7.89PCh. 7 - Prob. 7.90PCh. 7 - Prob. 7.91PCh. 7 - Prob. 7.92PCh. 7 - Prob. 7.93PCh. 7 - Prob. 7.94PCh. 7 - Prob. 7.95PCh. 7 - Prob. 7.96PCh. 7 - Prob. 7.97PCh. 7 - Prob. 7.98PCh. 7 - Prob. 7.99PCh. 7 - How many calories of heat are needed to increase...Ch. 7 - Prob. 7.101PCh. 7 - If it takes 37.0 cal of heat to raise the...Ch. 7 - Prob. 7.103PCh. 7 - What phase change is shown in the accompanying...Ch. 7 - Prob. 7.105PCh. 7 - Which process requires more energy, melting 250 g...Ch. 7 - Consider the cooling curve drawn below a. Which...Ch. 7 - Prob. 7.108PCh. 7 - Draw the heating curve that is observed when...Ch. 7 - Prob. 7.110PCh. 7 - Use the following values to answer each part. The...Ch. 7 - Prob. 7.112PCh. 7 - If you pack a bag of potato chips for a snack on a...Ch. 7 - Prob. 7.114PCh. 7 - Prob. 7.115PCh. 7 - Prob. 7.116PCh. 7 - Prob. 7.117PCh. 7 - If a scuba diver inhales 0.50 L of air at a depth...Ch. 7 - Prob. 7.119CPCh. 7 - As we learned in Chapter 5, an automobile airbag...
Knowledge Booster
Similar questions
- aIs the process of boiling water exothermic or endothermic with respect to the water? bA charged object is moved closer to another object that has the same charge. The energy of the system changes. Is it a change in kinetic energy or potential energy? Is the energy changes an increase or a decrease?arrow_forwardThe quicklime produced in Question 63 is frequently converted to calcium hydroxide, sometimes called slaked lime, by an exothermic reaction with water: CaO(s)+H2O(l)Ca(OH)2(s)+65.3kJ. What mass in grams of quicklime was processed in a reaction that transferred 291kJ of energy?arrow_forwardHow many joules of heat are lost by 3580 kg of granite asit cools from 41.2°C to -12.9°C? The specific heat ofgranite is 0.803J/(gC) .arrow_forward
- In the following equation for a chemical reaction, the notation s, l, or g indicates whether the substance is in the solid, liquid, or gaseous state:2H2S(g)+3O2(g)2H20(g)+2SO2(g)+energy. Identify each of the following as a product or reactant: a SO2(g); b H2S(g); c O2(g); d H20(g). When the reaction takes place, is energy released or absorbed? Is the reaction endothermic or exothermic?arrow_forwardAs a child plays on a swing, at what point in her movement is her kinetic energy the greatest? At what point is potential energy at its maximum?arrow_forwardHow much heat is required to raise the temperature of 100. grams of water from 25C near room temperature to 100.C its boiling point? The specific heat of water is approximately 4.2Jperg-K. a.3.2104J b.32J c.4.2104J d.76Jarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning


Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning