
Concept explainers
a)
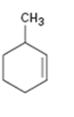
Interpretation:
The name of the cycloalkane shown is to be given.
Concept introduction:
The maximum number of carbons in the ring is counted. Based on the name of the parent cycloalkane–the cycloalkene is named with the suffix–ene. The cycloalkane is numbered such that the double bond is in between C1 & C2 and the first substituent has the lowest number possible. Usually the position of a double bond is not shown in the name because it is always between C1 & C2. In dienes and trienes, however the position of double bonds is shown.
To name:
The cycloalkane shown.
b)
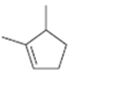
Interpretation:
The name of the cycloalkane shown is to be given.
Concept introduction:
The maximum number of carbons in the ring is counted. Based on the name of the parent cycloalkane–the cycloalkene is named with the suffix–ene. The cycloalkane is numbered such that the double bond is in between C1 & C2 and the first substituent has the lowest number possible. Usually the position of a double bond is not shown in the name because it is always between C1 & C2. In dienes and trienes, however the position of double bonds is shown.
To name:
The cycloalkane shown.
c)
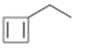
Interpretation:
The name of the cycloalkane shown to be given.
Concept introduction:
The maximum number of carbons in the ring is counted. Based on the name of the parent cycloalkane the cycloalkene is named with the suffix–ene. The cycloalkane is numbered such that the double bond is in between C1 & C2 and the first substituent has the lowest number possible. Usually the position of a double bond is not shown in the name because it is always between C1 & C2. In dienes and trienes, however the position of double bonds is shown.
To name:
The cycloalkane shown.
d)
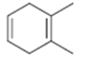
Interpretation:
The name the cycloalkane shown to be given.
Concept introduction:
The maximum number of carbons in the ring is counted. Based on the name of the parent cycloalkane–the cycloalkene is named with the suffix–ene. The cycloalkane is numbered such that the double bond is in between C1 & C2 and the first substituent has the lowest number possible. Usually the position of a double bond is not shown in the name because it is always between C1 & C2. In dienes and trienes, however the position of double bonds is shown.
To name:
The cycloalkane shown.
e)
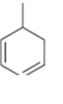
Interpretation:
The name the cycloalkane shown is to be given.
Concept introduction:
The maximum number of carbons in the ring is counted. Based on the name of the parent cycloalkane–the cycloalkene is named with the suffix–ene. The cycloalkane is numbered such that the double bond is in between C1 & C2 and the first substituent has the lowest number possible. Usually the position of a double bond is not shown in the name because it is always between C1 & C2. In dienes and trienes, however the position of double bonds is shown.
To name:
The cycloalkane shown
f)
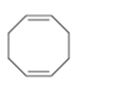
Interpretation:
The name of the cycloalkane shown is to be given.
Concept introduction:
The maximum number of carbons in the ring is counted. Based on the name of the parent cycloalkane–the cycloalkene is named with the suffix–ene. The cycloalkane is numbered such that the double bond is in between C1 & C2 and the first substituent has the lowest number possible. Usually the position of a double bond is not shown in the name because it is always between C1 & C2. In dienes and trienes, however the position of double bonds is shown.
To name:
The cycloalkane shown.
Trending nowThis is a popular solution!

Chapter 7 Solutions
Bundle: Organic Chemistry, Loose-leaf Version, 9th + LMS Integrated for OWLv2, 4 terms (24 months) Printed Access Card
- Draw all 12 acyclic (no rings) isomers of formula C4H7Br. Include stereoisomers.arrow_forwardname the following compounds, indicating stereochemistry, when applicable.arrow_forwardDraw the structures of the following molecules: (a) trans-l-Bromo-3-methylcyclohexane (b) cis-1, 2-Dimethylcyclobutane (c) trans-1 -tert-Butyl-2-ethylcyclohexanearrow_forward
- OChem HELP... Draw the conformational structures (chair or boat conformations) for the MAJOR product formed when 1-tert-butylcyclohexene reacts with each of the following reagents. Also, indicate if the product obtained is racemic form. a) Br2, CCl4 b) Br2, H2O c) OsO4, then aqueous NaHSO3 d) ICl e) mCPBA, then H3O+, H20 f) O3, then Me2S (conformational structure not required) g) BH3:THF, then H2O2, HO- h) D2, Pt i) Hg(OAc)2 in THF-H2O, then NaBH4, HO- j) BD3 :THF, then CH3CO2Tarrow_forwardIndicate what to do to obtain the compund (cyclohexylidenemethyl)cyclohexane from (C6H5)3P=CHCH2CH3arrow_forwardPredict the products of the following reactions. cyclopentadiene + methyl acrylate, CH2“CH¬COOCH3arrow_forward
- By hydrohalogenation of CHF2-CH=CH2 halogen atom is added to: Select one:a. second carbon atomb. Third carbon atomc. No reactiond. First carbon atomarrow_forwarddescribe Stability of Cycloalkenesarrow_forwardHow's is 7-oxabicyclo[4.1.0]heptane converted to (1R,2S)-1,2-dimethylcyclohexanearrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning


