
Concept explainers
a)
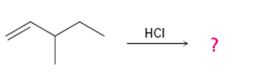
Interpretation:
The product and the complete arrow-pushing mechanism for the electrophilic reaction given which involves a carbocation rearrangement.
Concept introduction:
In electrophilic addition reactions, the first step is the attack of the π electrons of the double bond on the hydrogen of the
To predict:
The product and to show the complete arrow-pushing mechanism for the electrophilic reaction given which involves a carbocation rearrangement.
b)
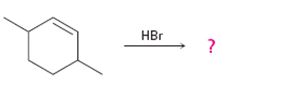
Interpretation:
The product and the complete arrow-pushing mechanism for the electrophilic reaction given which involves a carbocation rearrangement.
Concept introduction:
In electrophilic addition reactions, the first step is the attack of the π electrons of the double bond on the hydrogen of the alkyl halide to yield a carbocation. One of the carbon in C=C gets attached to hydrogen while the other acquires a positive charge. In the second step, the carbocation formed can rearrange to give another more stable carbocation either by a hydride shift (shift of hydrogen atom with its electron pair) or by an alkyl shift (shift of an alkyl group with its electron pair) between neighboring carbons. In the last step the carbocation produced reacts with the halide ion to give the alkyl halide.
To predict:
The product and to show the complete arrow-pushing mechanism for the electrophilic reaction given which involves a carbocation rearrangement.
c)

Interpretation:
The product and the complete arrow-pushing mechanism for the electrophilic reaction given which involves a carbocation rearrangement.
Concept introduction:
In electrophilic addition reactions, the first step is the attack of the π electrons of the double bond on the H+ of the acid to yield a carbocation. One of the carbon in C=C gets attached to hydrogen while the other acquires a positive charge. In the second step, the carbocation formed can rearrange to give a more stable carbocation either by a hydride shift (shift of hydrogen atom with its electron pair) or by an alkyl shift (shift of an alkyl group with its electron pair) between neighboring carbons. In the last step the carbocation produced reacts with the halide ion to give the alkyl halide.
To predict:
The product and to show the complete arrow-pushing mechanism for the electrophilic reaction given which involves a carbocation rearrangement.
Trending nowThis is a popular solution!

Chapter 7 Solutions
Bundle: Organic Chemistry, Loose-leaf Version, 9th + LMS Integrated for OWLv2, 4 terms (24 months) Printed Access Card
- A student proposes the following reaction mechanism for the reaction in Model 6. Which step inthis mechanism is least favorable? Explain your reasoning.arrow_forwardCan you help me predict this product and explain the mechanism? This problem is the carbocation rearrangements.arrow_forwardDraw the product of the reaction below, including step by step reaction mechanism with curly arrows and by-products.arrow_forward
- Predict the product, draw the mechanism, and plot the reaction coordinate diagram for theE1 reaction.arrow_forwardShow me the mechanism of this reaction using only 1 bromination. *R listed is NHCOCH3.arrow_forwardDo the reactions below proceed in good yield from left to right as shown? Please fill in Yes or Noarrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
