
Organic Chemistry
7th Edition
ISBN: 9780321826596
Author: Bruice, Paula Yurkanis/
Publisher: Pearson College Div
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 8, Problem 105P
The experiment shown next and discussed in Section 8.13 shows that the proximity of the chloride ion to C-2 in the transition state causes the 1,2·addition product to form more rapidly than the 1,4-addition product.
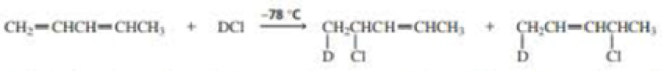
- a. Why was it important for the investigators to know that the preceding reaction was being carried out under kinetic control?
- b. How could the investigators know that the reaction was being carried out under kinetic control?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
A student wanted to know whether the greater proximity of the nucleophile to the C-2 carbon in the transition state is what causes the 1,2-addition product to be formed faster when 1,3-butadiene reacts with HCl. Therefore, she decided to investigate the reaction of 2-methyl-1,3-cyclohexadiene with HCl. Her friend told her that she should use 1-methyl-1,3-cyclohexadiene instead. Should she follow her friend’s advice?
1. Write the reaction that you will do this week for the Williamson ether synthesis. Label the nucleophile and the electrophile, the nucleophilic atom and the electrophilic atom.
2. Mechanistically, what type of reaction is the ether synthesis that you will perform this week?
3. An extraction is done for the work-up. What liquids will be used for the extraction? In which liquid will the product be? Will the product be in the top layer or the bottom layer?
How does changing the solvent from CH3OH to DMSO affect the rate of an E2 reaction?
Chapter 8 Solutions
Organic Chemistry
Ch. 8.1 - Prob. 1PCh. 8.1 - Prob. 2PCh. 8.4 - Prob. 3PCh. 8.5 - Prob. 5PCh. 8.6 - a. Predict the relative bond lengths of the three...Ch. 8.6 - Prob. 7PCh. 8.6 - Prob. 8PCh. 8.8 - Prob. 9PCh. 8.9 - Prob. 10PCh. 8.9 - Prob. 12P
Ch. 8.9 - Prob. 13PCh. 8.10 - Prob. 14PCh. 8.10 - What orbitals contain the electrons represented as...Ch. 8.10 - Prob. 16PCh. 8.10 - Prob. 17PCh. 8.11 - Prob. 18PCh. 8.11 - Prob. 19PCh. 8.11 - Prob. 20PCh. 8.12 - Prob. 21PCh. 8.12 - Prob. 22PCh. 8.12 - Prob. 23PCh. 8.13 - Prob. 24PCh. 8.13 - Prob. 25PCh. 8.13 - Prob. 26PCh. 8.14 - Prob. 27PCh. 8.14 - Prob. 28PCh. 8.14 - Prob. 29PCh. 8.15 - Which member of each pair is the stronger acid?Ch. 8.15 - Which member of each pair is the stronger base? a....Ch. 8.15 - Rank the following compounds from strongest acid...Ch. 8.15 - Prob. 34PCh. 8.16 - Prob. 35PCh. 8.17 - Prob. 37PCh. 8.17 - Prob. 38PCh. 8.17 - Prob. 39PCh. 8.17 - Prob. 40PCh. 8.17 - Prob. 41PCh. 8.17 - Prob. 42PCh. 8.18 - Prob. 43PCh. 8.18 - Prob. 44PCh. 8.18 - Prob. 45PCh. 8.18 - Prob. 47PCh. 8.19 - Prob. 48PCh. 8.19 - Prob. 49PCh. 8.19 - Prob. 50PCh. 8.19 - Prob. 51PCh. 8.19 - Prob. 52PCh. 8.19 - Prob. 53PCh. 8.19 - Prob. 55PCh. 8.20 - Prob. 56PCh. 8 - Prob. 57PCh. 8 - Prob. 58PCh. 8 - Prob. 59PCh. 8 - Prob. 60PCh. 8 - Prob. 61PCh. 8 - Prob. 62PCh. 8 - Prob. 63PCh. 8 - Prob. 64PCh. 8 - Prob. 65PCh. 8 - Prob. 66PCh. 8 - Prob. 67PCh. 8 - Prob. 68PCh. 8 - Prob. 69PCh. 8 - Which compound is the strongest base?Ch. 8 - Prob. 71PCh. 8 - Prob. 72PCh. 8 - Prob. 73PCh. 8 - Prob. 74PCh. 8 - Prob. 75PCh. 8 - Prob. 76PCh. 8 - Prob. 77PCh. 8 - Prob. 78PCh. 8 - Purine is a heterocyclic compound with four...Ch. 8 - Prob. 80PCh. 8 - Why is the delocalization energy of pyrrole (21...Ch. 8 - Prob. 82PCh. 8 - Prob. 83PCh. 8 - Prob. 84PCh. 8 - A student obtained two products from the reaction...Ch. 8 - Prob. 86PCh. 8 - a. How could each of the following compounds be...Ch. 8 - Draw the products obtained from the reaction of...Ch. 8 - How would the following substituents affect the...Ch. 8 - Prob. 90PCh. 8 - The acid dissociation constant (Ka) for loss of a...Ch. 8 - Protonated cyclohexylamine has a Ka = 1 1011...Ch. 8 - Prob. 93PCh. 8 - Prob. 94PCh. 8 - Prob. 95PCh. 8 - Prob. 96PCh. 8 - Prob. 97PCh. 8 - a. Propose n mechanism for the following reaction:...Ch. 8 - Prob. 99PCh. 8 - As many as 18 different Diels-Alder products can...Ch. 8 - Prob. 101PCh. 8 - Prob. 102PCh. 8 - Prob. 103PCh. 8 - Prob. 104PCh. 8 - The experiment shown next and discussed in Section...Ch. 8 - Prob. 106PCh. 8 - Prob. 107PCh. 8 - Prob. 108PCh. 8 - Prob. 1PCh. 8 - Prob. 2PCh. 8 - Prob. 3PCh. 8 - Prob. 4PCh. 8 - Prob. 5PCh. 8 - Prob. 6PCh. 8 - Prob. 7PCh. 8 - Prob. 8PCh. 8 - Prob. 9PCh. 8 - Prob. 10PCh. 8 - Prob. 11PCh. 8 - Prob. 12P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Fill in the reagents below/above the arrow in the ff reactionarrow_forwardThe SN1 reaction is a two-step reaction, and the first step of this reaction is the formation of a carbocation intermediate. Draw the carbocation intermediates that form from the alkyl chlorides in tubes 8 and 15. 2. Again, use tubes 8 and 15 to answer the following question. In general, the more stable carbocation forms faster. Given this, which carbocation is more stable, or do they have the same stability?arrow_forwardWhere is the p-tertbuttlphenol that should be in breaker B explain what happened.arrow_forward
- A student wanted to know whether the greater proximity of the ucleophile to the C-2 carbon in the transition state is what causes the 1,2-addition product to be formed faster when 1,3-butadiene reacts with HCl. Therefore, she decided to investigate the reaction of 2-methyl-1,3-cyclohexadiene with HCl.Her friend told her that she should use 1-methyl-1,3-cyclohexadiene instead. Should she follow her friend’s advice?arrow_forwardHow does doubling [B:] affect the rate of an E1 reaction?arrow_forwardWhy we need step 3 before step 4? a. Because the nitro group increases the electrophilicity at the ortho positions which is where the bromine is added. b. Because the amine group is a strong ortho, para director which is what controls the regiochemical outcome of this bromination. c. Step 4 is unessesary. The symmetry of compound 3 allows for the bromination to be regioselective and give compound 5. 5. There will be a mixture of products because there is no selectivity for a major product.arrow_forward
- What are the important mechanisms that should be highlighted in this image? How do they work together to create the final product?arrow_forwardIn the reaction series below, write down the appropriate reagents that can be used where there are question marks.arrow_forwardWhy does increasing alkyl substitution increase the rate of an E2 reaction?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Enzymes - Effect of cofactors on enzyme; Author: Tutorials Point (India) Ltd;https://www.youtube.com/watch?v=AkAbIwxyUs4;License: Standard YouTube License, CC-BY
Enzyme Catalysis Part-I; Author: NPTEL-NOC IITM;https://www.youtube.com/watch?v=aZE740JWZuQ;License: Standard Youtube License