
Organic Chemistry - Standalone book
10th Edition
ISBN: 9780073511214
Author: Francis A Carey Dr., Robert M. Giuliano
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 8, Problem 49P
Identify compounds A and B in the retrosynthesis shown and use this information to design a synthesis of the desired nitrile showing all necessary reagents.
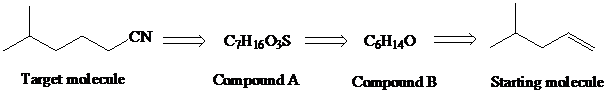
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
draw out the retrosynthesis analysis for the product shown using ketones, and grignard reagents, going back to the alkyl or aryl halide, give three possible pathways
Fill in the missing compounds in the partial retrosynthesis shown and devise a synthesis showing all necessary reagents based on your retrosynthesis.
Complete the following multi step synthesis by showing the major intermediate products and the reagents nessesary for each step
Chapter 8 Solutions
Organic Chemistry - Standalone book
Ch. 8.1 - What three alkenes yield 2-methylbutane on...Ch. 8.2 - Prob. 2PCh. 8.2 - Prob. 3PCh. 8.3 - Prob. 4PCh. 8.4 - Prob. 5PCh. 8.4 - Give a structural formula for the carbocation...Ch. 8.5 - Prob. 7PCh. 8.6 - Instead of the three-step process of Mechanism...Ch. 8.6 - The rates of hydration of the two alkenes shown...Ch. 8.6 - Is the electrophilic addition of hydrogen chloride...
Ch. 8.7 - You can calculate the equilibrium constant for the...Ch. 8.7 - Does the presence or absence of a catalyst such as...Ch. 8.7 - The gas phase reaction of ethanol with hydrogen...Ch. 8.8 - Prob. 14PCh. 8.8 - Hydroborationoxidation of -pinene, like its...Ch. 8.10 - Arrange the compounds 2-methyl-1-butene,...Ch. 8.10 - Give the structure of the product formed when each...Ch. 8.11 - Prob. 18PCh. 8.11 - Prob. 19PCh. 8.12 - Prob. 20PCh. 8.12 - Prob. 21PCh. 8.13 - Prob. 22PCh. 8.14 - Prob. 23PCh. 8.14 - Prob. 24PCh. 8 - How many alkenes yield...Ch. 8 - Prob. 26PCh. 8 - Catalytic hydrogenation of...Ch. 8 - Prob. 28PCh. 8 - Prob. 29PCh. 8 - Prob. 30PCh. 8 - Prob. 31PCh. 8 - A single epoxide was isolated in 7984% yield in...Ch. 8 - Prob. 33PCh. 8 - Prob. 34PCh. 8 - On catalytic hydrogenation over a rhodium...Ch. 8 - Prob. 36PCh. 8 - Prob. 37PCh. 8 - Prob. 38PCh. 8 - Prob. 39PCh. 8 - 1-Butene has a higher heat of hydrogenation than...Ch. 8 - Match the following alkenes with the appropriate...Ch. 8 - The heats of reaction were measured for addition...Ch. 8 - Complete the following table by adding + and -...Ch. 8 - Match the heats of hydrogenation (107 kJ/mol,...Ch. 8 - The iodination of ethylene at 25 C is...Ch. 8 - Specify reagents suitable for converting...Ch. 8 - (a) Which primary alcohol of molecular formula...Ch. 8 - Identify compounds A and B in the retrosynthesis...Ch. 8 - Identify compounds A and B in the retrosynthesis...Ch. 8 - Prob. 50PCh. 8 - On being heated with a solution of sodium ethoxide...Ch. 8 - Compound A (C7H15Br) is not a primary alkyl...Ch. 8 - Prob. 53PCh. 8 - Prob. 54PCh. 8 - A mixture of three alkenes (A, B, and C) was...Ch. 8 - Reaction of 3,3-dimethyl-1-butene with hydrogen...Ch. 8 - Dehydration of 2,2,3,4,4-pentamethyl-3-pentanol...Ch. 8 - Prob. 58PCh. 8 - East Indian sandalwood oil contains a hydrocarbon...Ch. 8 - Prob. 60PCh. 8 - Prob. 61PCh. 8 - Prob. 62PCh. 8 - Prob. 63PCh. 8 - Prob. 64PCh. 8 - On the basis of the mechanism of acid-catalyzed...Ch. 8 - As a method for the preparation of alkenes, a...Ch. 8 - Which of the following is the most reasonable...Ch. 8 - Prob. 68PCh. 8 - Oxymercuration Concerns about mercurys toxicity...Ch. 8 - Prob. 70DSPCh. 8 - Prob. 71DSPCh. 8 - Prob. 72DSPCh. 8 - Prob. 73DSPCh. 8 - Oxymercuration Concerns about mercurys toxicity...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Show how to prepare a,b-unsaturated aldehyde by an aldol reaction followed by dehydration of the aldol product.arrow_forwardComplete the following multistep syntheses by showing the major products and the reagents nessesary for each steparrow_forward1 Using this retrosynthesis scheme, Show a forward synthesis with all relevant reagents and conditions needed to make the desired molecule. Don't show mechanism arrows or intermediates.arrow_forward
- show the mechanism of the following reaction step by step. Via Fischer esterification and transesterification.arrow_forwardPropose a multistep synthetic route to the following structure that includes all reagents, substrates, as well as the products in each step.arrow_forwardWhich reaction sequence would provide the following transformation?arrow_forward
- Which reaction sequence would accomplish this transformation?arrow_forwardProvide a reasonable multistep synthesis of the following molecule from the indicated starting material, using any reagents necessary. Please include both a retrosynthesis and a forward synthesis (that includes the product and reagents of each individual step in your synthesis).arrow_forwardThe following synthesis requires more than one step. Specify the reagents you would use to carry it out.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
How to Design a Total Synthesis; Author: Chemistry Unleashed;https://www.youtube.com/watch?v=9jRfAJJO7mM;License: Standard YouTube License, CC-BY