
Concept explainers
Interpretation:
The retrosynthesis analysis and the actual synthesis are to be written for the preparation of each target molecule from the given starting molecule.
Concept introduction:
>The retrosynthesis is a reaction that involves
on acid catalyzed dehydration, an alcohol gives the corresponding more substituted alkene as the major product.
>The alkene, on reaction with halogen in water, undergoes addition of halogen and hydroxyl group across the double bond in a way that the halogen atom is bonded to the less substituted double bonded carbon atom and hydroxyl group is bonded to the more substituted double bonded carbon atom.
>Alkenes react with hydrogen halide and yield haloalkane.
>Alkenes, on hydration, form more substituted alcohol.
>Alkenes, on hydroboration-oxidation, form less substituted alcohol.
>Alkenes, in presence of peroxy acid, undergo
In presence of a strong base,
Answer to Problem 50P
Solution:
The retrosynthetic analysis and the actual synthesis for the preparation of each target molecule from the given starting compound are as follows:
a)
Retrosynthesis:
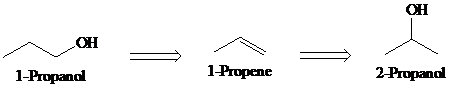
Synthesis:
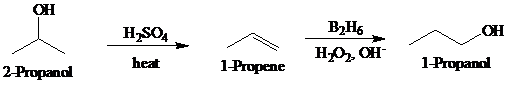
b)
Retrosynthesis:
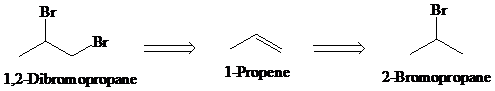
Synthesis:
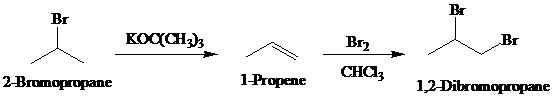
c)
Retrosynthesis:
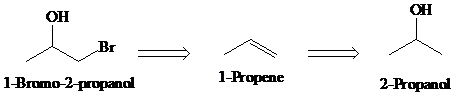
Synthesis:
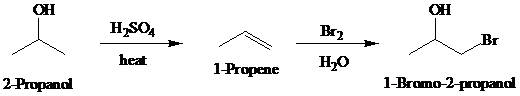
d)
Retrosynthesis:
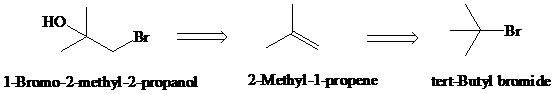
Synthesis:
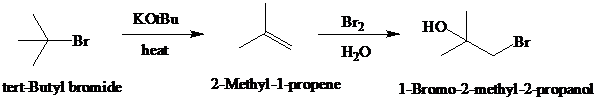
e)
Retrosynthesis:
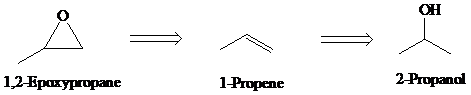
Synthesis:

f)
Retrosynthesis:
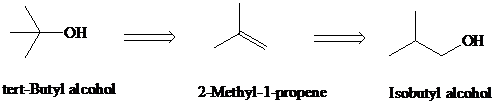
Synthesis:

g)
Retrosynthesis:
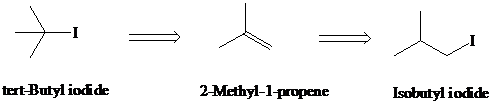
Synthesis:

h)
Retrosynthesis:
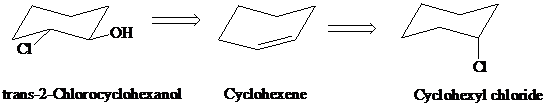
Synthesis:

Explanation of Solution
a)
Retrosynthetic analysis for the target molecule
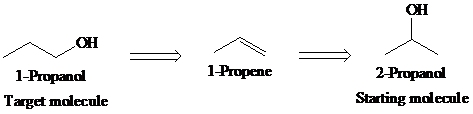
The actual synthetic route for the above retrosynthesis is as follows:
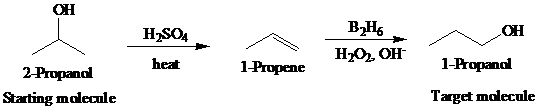
b)
Retrosynthetic analysis for the target molecule
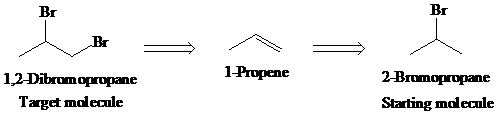
The actual synthetic route for the above retrosynthesis is as follows:
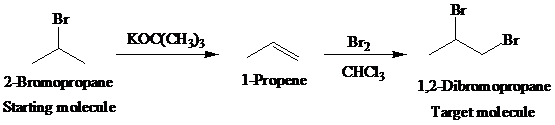
c)
Retrosynthetic analysis for the target molecule
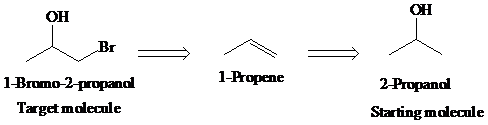
The actual synthetic route for the above retrosynthesis is as follows:
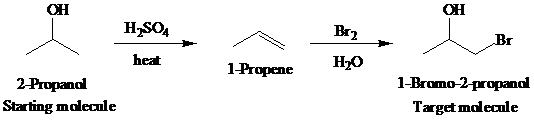
d)
Retrosynthetic analysis for the target molecule
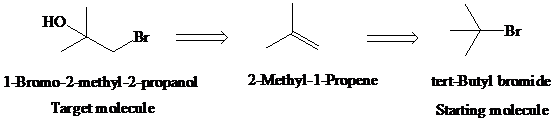
The actual synthetic route for the above retrosynthesis is as follows:
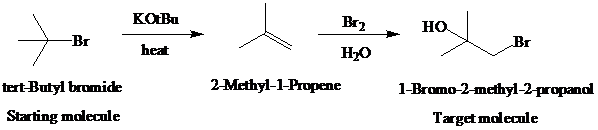
e)
Retrosynthetic analysis for the target molecule
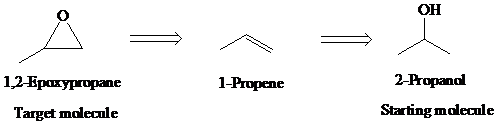
The actual synthetic route for the above retrosynthesis is as follows:
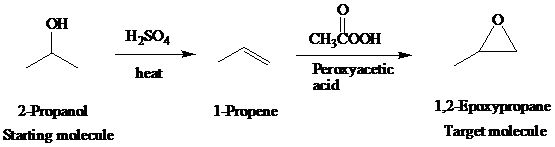
f)
Retrosynthetic analysis for the target molecule
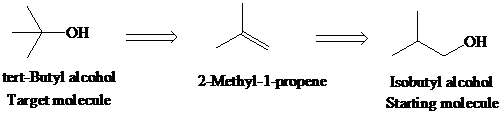
The actual synthetic route for the above retrosynthesis is as follows:
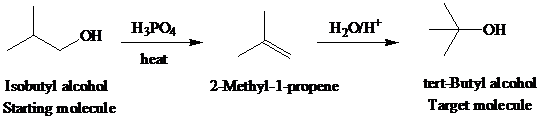
g)
Retrosynthetic analysis for the target molecule
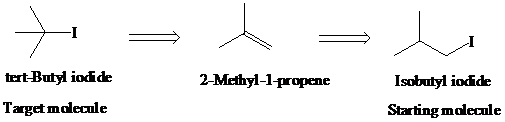
The actual synthetic route for the above retrosynthesis is as follows:
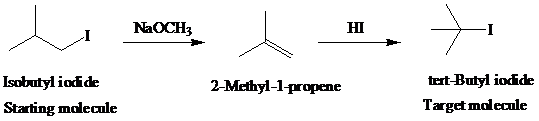
h)
Retrosynthetic analysis for the target molecule

The actual synthetic route for the above retrosynthesis is as follows:

Therefore, the retrosynthesis and synthesis reactions for the given compounds were proposed.
Want to see more full solutions like this?
Chapter 8 Solutions
ORGANIC CHEMISTRY-W/STUD.SOLN.MAN.
- Beginning with phenylacetylene, outline a method to synthesize the following secondary alcohol, necessitating a sequence of synthetic stages. You are permitted to employ any reactants from any part of your organic chemistry coursework; however, if any reactant contributes additional carbon atoms, it must initially be in the form of an alcohol (for instance, if you intend to use propyl bromide, you must first derive it from propanol before proceeding).arrow_forwardAcetal formation is a characteristic reaction of aldehydes and ketones, but not of carboxylic acids. Use retrosynthetic analysis to show how you could use a cyclic acetal protecting group in the following synthesis, then write equations for the procedure showing the necessary reagents.arrow_forwardWittig reactions with the following -chloroethers can be used for the synthesis of aldehydes and ketones. (a) Draw the structure of the triphenylphosphonium salt and Wittig reagent formed from each chloroether. (b) Draw the structural formula of the product formed by treating each Wittig reagent with cyclopentanone. Note that the functional group is an enol ether or, alternatively, a vinyl ether. (c) Draw the structural formula of the product formed on acid-catalyzed hydrolysis of each enol ether from part (b).arrow_forward
- ORGANIC Chemistry Which are common names for carboxylic acid derivatives? A. Esters B. Ethers C. Formic Acid D. Acetic Anhydride Answers: A. C, D, and A B. D and B only C. C and D only D. None I think it is A, please answer and explain why. Thank youarrow_forwardSynthetize 3-pentynal from 3-butynal using whatever organic/inorganic reagents are needed.arrow_forwardAnswer all three parts plz. You are starting with three aryl bromides. They undergo a typical grignard reaction with magnesium metal and anhydrous ether (diethyl ether). Dry ice is then added to it and it is then cooled. Draw and label the names of the aryl carboxylic acids that will form for each. Part a) bromobenzene Part b) para-bromoanisole Part c) meta-bromoanisolearrow_forward
- Use a series of reactions to show how you can make 2-pentanone and 3-pentanone from 2-pentyne. Name all reactants and intermediate products formed. Indicate the type(s) of reactions required.arrow_forwardBy means of a series of equations outline a synthesis for each of the compounds from the indicated starting material. You may use any other reagents needed.a. propionyl chloride from n-propyl alcoholb. n-butyric anhydride from n-butyl alcoholc. sec-butyl acetate from 2-butened. ethyl butanoate from n-butyl alcohole. benzamide from toluenearrow_forwardWhy is it not advisable to use aqueous hydrochloric acid in a Grignard reaction of a ketone? A) The Grignard reagent will react with the acid and cannot react with the ketone. B) The ketone will be protonated and will become unreactive. C) The ketone will form an unreactive enol. D) The Grignard reagent won't dissolve in aqueous solutionsarrow_forward
- Design a synthesis for each of the following compounds, usingany inorganic reagent of your choice and any hydrocarbon oralkyl halide of your choice:a. Octanalb. Cyclohexanonec. 2-Phenylethanoic acidarrow_forward1. Include reagents 2. Include mechanisms usedarrow_forwardWhich of the following synthetic routes will convert 3-methyl-2-butanol into 3-methyl-1-butanol?arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

