
Concept explainers
Interpretation:
The pH values after the addition of each proportion of the base to the acid is to be determined. Also, the titration curve needs to be drawn.
Concept introduction:
Titration curve is drawn to determine the change in pH of an acid or base with respect to the added volume of base or acid to it.
The titration curve can be drawn between a strong/weak acid and strong/weak base. The change in pH shows different patterns for different combinations of acids and bases.
Explanation of Solution
Initial pH of the analyte solution can be determined as follows:
Propanoic acid is a weak acid that forms equilibrium when dissolved in water. The equilibrium is as follows.
The amount of acid at the beginning
| Reaction | Proanoic acid | Propanoate ion | OH- |
| Initial | 0.1 | 0 | 0 |
| Change | -x | +x | +x |
| Equilibrium | (0.1-x) | x | x |
The acid dissociation constant can be represented as follows:
Solving this quadratic equation gives the amount of hydrogen ions in the solution.
On solving the only possible value of x is
Now, pH can be calculated as follows:
Addition of
Total amount of acid to be neutralized
Amount of base added
Then the ICE table after the addition of base is created in order to determine the pH of the solution using Henderson-Hasselbalch equation.
| Reaction | Propanoic acid | OH- | Propanoate ion | H+ |
| Initial | 0.0025 | 0 | 0 | 0 |
| Add | 0 | 0.0004 | ||
| Change | -0.0004 | -0.0004 | 0.0004 | 0.0004 |
| Equilibrium | 0.0021 | 0 | 0.0004 | 0.0004 |
Concentration of base after addition of acid
Concentration of ammonium ion
In the Henderson-Hasselbalch equation, the pKa is used.
Applying the Henderson-Hasselbalch equation,
Addition of
Total amount of acid to be neutralized
Amount of base added
Then the ICE table after the addition of base is created in order to determine the pH of the solution using Henderson-Hasselbalch equation.
| Reaction | Propanoic acid | OH- | Propanoate ion | H+ |
| Initial | 0.0025 | 0 | 0 | 0 |
| Add | 0 | 0.0008 | ||
| Change | -0.0008 | -0.0008 | 0.0008 | 0.0008 |
| Equilibrium | 0.0017 | 0 | 0.0008 | 0.0008 |
Concentration of acid after addition of base
Concentration of propanoate ion
Applying the Henderson-Hasselbalch equation,
Addition of
Total amount of acid to be neutralized
Amount of base added
Then the ICE table after the addition of base is created in order to determine the pH of the solution using Henderson-Hasselbalch equation.
| Reaction | Propanoic acid | OH- | Propanoate ion | H+ |
| Initial | 0.0025 | 0 | 0 | 0 |
| Add | 0 | 0.00125 | ||
| Change | -0.00125 | -0.00125 | 0.00125 | 0.00125 |
| Equilibrium | 0.00125 | 0 | 0.00125 | 0.00125 |
Concentration of acid after addition of base
Concentration of propanoate ion
Applying the Henderson-Hasselbalch equation,
Addition of
Total amount of acid to be neutralized
Amount of base added
Then the ICE table after the addition of base is created in order to determine the pH of the solution using Henderson-Hasselbalch equation.
| Reaction | Propanoic acid | H+ | Propanoate ion | OH- |
| Initial | 0.0025 | 0 | 0 | 0 |
| Add | 0 | 0.002 | ||
| Change | -0.002 | -0.002 | 0.002 | 0.002 |
| Equilibrium | 0.0005 | 0 | 0.002 | 0.002 |
Concentration of acid after addition of base
Concentration of propanoate ion
Applying the Henderson-Hasselbalch equation,
Addition of
Total amount of acid to be neutralized
Amount of base added
Then the ICE table after the addition of base is created in order to determine the pH of the solution using Henderson-Hasselbalch equation.
| Reaction | Propanoic | OH- | Propanoate | H+ |
| Initial | 0.0025 | 0 | 0 | 0 |
| Add | 0 | 0.0024 | ||
| Change | -0.0024 | -0.0024 | 0.0024 | 0.0024 |
| Equilibrium | 0.0001 | 0 | 0.0024 | 0.0024 |
Concentration of acid after addition of base
Concentration of propanoate ion
Applying the Henderson-Hasselbalch equation,
Addition of
Total amount of acid to be neutralized
Amount of base added
Then the ICE table after the addition of base is created in order to determine the pH of the solution using Henderson-Hasselbalch equation.
| Reaction | Propanoic | OH- | Propanoate | H+ |
| Initial | 0.0025 | 0 | 0 | 0 |
| Add | 0 | 0.00245 | ||
| Change | -0.00245 | -0.00245 | -0.00245 | -0.00245 |
| Equilibrium | 0.00005 | 0 | -0.00245 | -0.00245 |
Concentration of acid after addition of base
Concentration of propanoate ion
Applying the Henderson-Hasselbalch equation,
Addition of
Total amount of acid to be neutralized
Amount of base added
Then the ICE table after the addition of base is created in order to determine the pH of the solution using Henderson-Hasselbalch equation.
| Reaction | Propanoic | OH- | Propanoate | H+ |
| Initial | 0.0025 | 0 | 0 | 0 |
| Add | 0 | 0.00249 | ||
| Change | -0.00249 | -0.00249 | -0.00249 | -0.00249 |
| Equilibrium | 0.00001 | 0 | -0.00249 | -0.00249 |
Concentration of acid after addition of base
Concentration of propanoate ion
Applying the Henderson-Hasselbalch equation,
Addition of
Total amount of acid to be neutralized
Amount of base added
Then the ICE table after the addition of base is created in order to determine the pH of the solution using Henderson-Hasselbalch equation.
| Reaction | Propanoic | OH- | Propanoate | H+ |
| Initial | 0.0025 | 0 | 0 | 0 |
| Add | 0 | 0.0025 | ||
| Change | -0.0025 | -0.0025 | -0.0025 | -0.0025 |
| Equilibrium | 0.0000 | 0 | -0.0025 | -0.0025 |
Concentration of acid after addition of base
Concentration of propanoate ion
At this point, there is no excess acid or base. Therefore, the only possible reaction here is the dissociation of the conjugate acid of the propanoic acid (that is propanoate ion).
Thereafter, using the Kb value for propanoate ion, the amount of hydrogen ions in the solution can be determined to get the pH value at this point.
| Reaction | Propanoate ion | Propanoic acid | OH- |
| Initial | 0.05 | 0 | 0 |
| Change | -X | +x | +x |
| Equilibrium | (0.05-x) | x | x |
Then the pH can be calculated as follows:
On solving the equation, the only possible value of x will be:
This is the concentration of hydroxide ion. The pOH value can be calculated as follows:
Thus, pH of the solution is
Addition of
Total amount of acid to be neutralized
Amount of base added
Then the ICE table after the addition of base is created in order to determine the pH of the solution using Henderson-Hasselbalch equation.
| Reaction | Propanoic | OH- | Propanoate | H+ |
| Initial | 0.0025 | 0 | 0 | 0 |
| Add | 0 | 0.0028 | ||
| Change | -0.0025 | 0.0025 | 0 | 0 |
| Equilibrium | 0 | 0.0003 | 0 | 0 |
Concentration of hydroxide ion
Addition of
Total amount of acid to be neutralized
Amount of base added
Then the ICE table after the addition of base is created in order to determine the pH of the solution using Henderson-Hasselbalch equation.
| Reaction | Propanoic | OH- | Propanoate | H+ |
| Initial | 0.0025 | 0 | 0 | 0 |
| Add | 0 | 0.0030 | ||
| Change | -0.0025 | 0.0025 | 0 | 0 |
| Equilibrium | 0 | 0.0005 | 0 | 0 |
Concentration of hydroxide ion
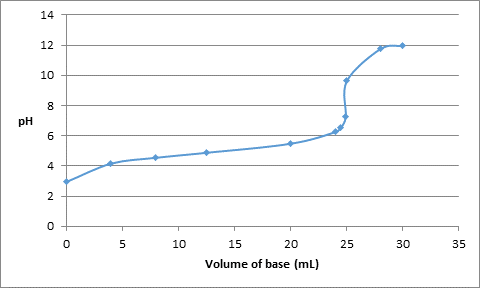
Thus, the pH values for volume of base added are as follows:
| Volume of base added (mL) | pH |
| 0.0 | 2.94 |
| 4.0 | 4.16 |
| 8.0 | 4.55 |
| 12.5 | 4.88 |
| 20.0 | 5.48 |
| 24.0 | 6.26 |
| 24.5 | 6.57 |
| 24.9 | 7.26 |
| 25.0 | 9.64 |
| 28.0 | 11.75 |
| 30.0 | 11.96 |
The titration curve can be drawn as follows:
Want to see more full solutions like this?
Chapter 8 Solutions
Chemical Principles
- Given the acid-base indicators in Question 37, select a suitable indicator for the following titrations. (a) sodium formate (NaCHO2) with HNO3 (b) hypochlorous acid with barium hydroxide (c) nitric acid with HI (d) hydrochloric acid with ammoniaarrow_forwardCalculate the maximum concentration of Mg2+ (molarity) that can exist in a solution of pH 12.00.arrow_forwardPhenol, C6H5OH, is a weak organic acid. Suppose 0.515 g of the compound is dissolved in enough water to make 125 mL of solution. The resulting solution is titrated with 0.123 M NaOH. C6H5OH(aq) + OH(aq) C6H5O(aq) + H2O() (a) What is the pH of the original solution of phenol? (b) What are the concentrations of all of the following ions at the equivalence point: Na+, H3O+, OH, and C6H5O? (c) What is the pH of the solution at the equivalence point?arrow_forward
- Repeat the procedure in Exercise 61, but for the titration of 25.0 mL of 0.100 M propanoic acid (HC3H5O2,Ka = 1.3 105) with 0.100 M NaOH.arrow_forwardWhat is the pH of a solution obtained by mixing 235 mL of NaOH with a pH of 11.57 and 316 mL of Sr(OH)2 with a pH of 12.09? Assume that volumes are additive.arrow_forwardUsing the diagrams shown in Problem 10-117, which of the solutions would have the greatest buffer capacity, that is, greatest protection against pH change, when the following occurs? a. A strong acid is added to the solution. b. A strong base is added to the solution.arrow_forward
- Estimate the pH that results when the following two solutions are mixed. a) 50 mL of 0.3 M CH3COOH and 50 mL of 0.4 M KOH b) 100 mL of 0.3 M CH3COOH and 50 mL of 0.4 M NaOH c) 150 mL of 0.3 M CH3COOH and 100 mL of 0.3 M Ba(OH)2 d) 200 mL of 0.3 M CH3COOH and 100 mL of 0.3 M Ba(OH)2arrow_forwardA solution made up of 1.0 M NH3 and 0.50 M (NH4)2SO4 has a pH of 9.26. a Write the net ionic equation that represents the reaction of this solution with a strong acid. b Write the net ionic equation that represents the reaction of this solution with a strong base. c To 100. mL of this solution, 10.0 mL of 1.00 M HCl is added. How many moles of NH3 and NH4+ are present in the reaction system before and after the addition of the HCl? What is the pH of the resulting solution? d Why did the pH change only slightly upon the addition of HCl?arrow_forwardWhen might a pH meter be better than an indicator to determine the end point of an acid-base titration?arrow_forward
- A solution of acetic acid, HC2H3O2, on a laboratory shelf was of undetermined concentration. If the pH of the solution was found to be 2.57, what was the concentration of the acetic acid? The Ka of acetic acid is 1.7 105.arrow_forwardConsider a solution prepared by mixing a weak acid HA and HCl. What are the major species? Explain what is occurring in solution. How would you calculate the pH? What if you added NaA to this solution? Then added NaOH?arrow_forwardA 1000.-mL solution of hydrochloric acid has a pH of 1.3. Calculate the mass (g) of HCl dissolved in the solution.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning





