
Chemistry (7th Edition)
7th Edition
ISBN: 9780321943170
Author: John E. McMurry, Robert C. Fay, Jill Kirsten Robinson
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 8, Problem 8.66SP
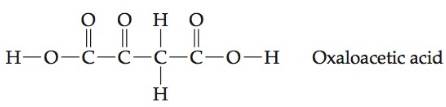
Oxaloacetic acid is an intermediate involved in the citric acid cycle of food
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
What is the hybridization of the indicated carbon atoms?
For each of the following molecules, write the Lewis structures, predict the electron geometry, molecular geometry, bond angles,
expected hybrid orbitals on central atoms and predict the overall polarity.
BRF3
ICI4
Chapter 8 Solutions
Chemistry (7th Edition)
Ch. 8 - Prob. 8.1PCh. 8 - Prob. 8.2ACh. 8 - PRACTICE 8.3 Acetic acid, CH3CO2H , is the main...Ch. 8 - APPLY 8.4 Benzene, C6H6 , is a cyclic molecule in...Ch. 8 - PRACTICE 8.5 Identify the orbitals that overlap to...Ch. 8 - APPLY 8.6 Describe the bonding in propane, C3H8 ,...Ch. 8 - PRACTICE 8.7 Describe the hybridization of the...Ch. 8 - APPLY 8.8 Describe the hybridization of each...Ch. 8 - PRACTICE 8.9 Describe the hybridization of the...Ch. 8 - APPLY 8.10 Describe the hybridization of the...
Ch. 8 - Prob. 8.11PCh. 8 - Conceptual APPLY 8.12 Match the following...Ch. 8 - Prob. 8.13PCh. 8 - Prob. 8.14ACh. 8 - Prob. 8.15PCh. 8 - Prob. 8.16ACh. 8 - Prob. 8.17PCh. 8 - Prob. 8.18ACh. 8 - Prob. 8.19PCh. 8 - Prob. 8.20ACh. 8 - Prob. 8.21PCh. 8 - Prob. 8.22ACh. 8 - PRACTICE 8.23 Draw two resonance structures for...Ch. 8 - APPLY 8.24 Draw two resonance structures for the...Ch. 8 - Prob. 8.25PCh. 8 - Prob. 8.26PCh. 8 - PROBLEM 8.27 Identify which of the following...Ch. 8 - Prob. 8.28PCh. 8 - Prob. 8.29PCh. 8 - Prob. 8.30PCh. 8 - Prob. 8.31CPCh. 8 - Prob. 8.32CPCh. 8 - Prob. 8.33CPCh. 8 - Prob. 8.34CPCh. 8 - Prob. 8.35CPCh. 8 - Prob. 8.36CPCh. 8 - Prob. 8.37CPCh. 8 - Prob. 8.38CPCh. 8 - Prob. 8.39CPCh. 8 - Prob. 8.40CPCh. 8 - Two dichioroethylene molecules with the same...Ch. 8 - Prob. 8.42SPCh. 8 - Prob. 8.43SPCh. 8 - Prob. 8.44SPCh. 8 - How many charge clouds are there around the...Ch. 8 - Prob. 8.46SPCh. 8 - Prob. 8.47SPCh. 8 - What shape do you expect for each of the following...Ch. 8 - Prob. 8.49SPCh. 8 - Prob. 8.50SPCh. 8 - Prob. 8.51SPCh. 8 - Prob. 8.52SPCh. 8 - Prob. 8.53SPCh. 8 - Acrylonitrile is used as the starting material for...Ch. 8 - Predict values for all bond angles in dimethyl...Ch. 8 - Oceanographers study the mixing of water masses by...Ch. 8 - Prob. 8.57SPCh. 8 - Prob. 8.58SPCh. 8 - Prob. 8.59SPCh. 8 - Prob. 8.60SPCh. 8 - Prob. 8.61SPCh. 8 - Prob. 8.62SPCh. 8 - Prob. 8.63SPCh. 8 - Prob. 8.64SPCh. 8 - Prob. 8.65SPCh. 8 - Oxaloacetic acid is an intermediate involved in...Ch. 8 - The atoms in the amino acid glycine are connected...Ch. 8 - Prob. 8.68SPCh. 8 - Prob. 8.69SPCh. 8 - Prob. 8.70SPCh. 8 - 8.71 What is the difference between London...Ch. 8 - 8.72 What are the most important kinds of...Ch. 8 - Of the substances Xe,CH3Cl,HF, which has: (a) The...Ch. 8 - 8.74 Methanol boils nearlyhigher than methane, but...Ch. 8 - Prob. 8.75SPCh. 8 - Prob. 8.76SPCh. 8 - Prob. 8.77SPCh. 8 - Prob. 8.78SPCh. 8 - Prob. 8.79SPCh. 8 - Prob. 8.80SPCh. 8 - 8.81 Draw three-dimensional structures of PCl3 and...Ch. 8 - Prob. 8.82SPCh. 8 - Prob. 8.83SPCh. 8 - Prob. 8.84SPCh. 8 - Prob. 8.85SPCh. 8 - 8.86 A liquid sample contains methylamine (CH3NH2)...Ch. 8 - Prob. 8.87SPCh. 8 - Prob. 8.88SPCh. 8 - Prob. 8.89SPCh. 8 - Prob. 8.90SPCh. 8 - Prob. 8.91SPCh. 8 - Prob. 8.92SPCh. 8 - Prob. 8.93SPCh. 8 - Prob. 8.94SPCh. 8 - Prob. 8.95SPCh. 8 - Prob. 8.96SPCh. 8 - Prob. 8.98CPCh. 8 - Prob. 8.99CPCh. 8 - Prob. 8.100CPCh. 8 - Prob. 8.101CPCh. 8 - Prob. 8.102CPCh. 8 - Prob. 8.103CPCh. 8 - Prob. 8.104CPCh. 8 - Prob. 8.105CPCh. 8 - Prob. 8.106CPCh. 8 - Prob. 8.107CPCh. 8 - Prob. 8.108CPCh. 8 - Prob. 8.109CPCh. 8 - The odor of cinnamon oil is due to cinnamaldehyde,...Ch. 8 - Prob. 8.111CPCh. 8 - Prob. 8.112CPCh. 8 - Prob. 8.113CPCh. 8 - Prob. 8.114CPCh. 8 - Prob. 8.115CPCh. 8 - Prob. 8.116CPCh. 8 - Prob. 8.117CPCh. 8 - Prob. 8.118CPCh. 8 - Prob. 8.119MPCh. 8 - Prob. 8.120MPCh. 8 - Prob. 8.121MP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- • explain the formation of multiple bonds in terms of the overlap of a combination of hybridized and unhybridized atomic orbitals.arrow_forwardAspirin, or acetylsalicylic acid, has the formula C9H8O4 and the skeleton structure (a) Complete the Lewis structure and give the number of bonds and bonds in aspirin. (b) What is the hybridization about the CO2H carbon atom (colored blue)? (c) What is the hybridization about the carbon atom in the benzene-like ring that is bonded to an oxygen atom (colored red)? Also, what is the hybridization of the oxygen atom bonded to this carbon atom?arrow_forwardIn propene CH3CH=CH2, the first carbon has sp3 hybrid orbitals and the second carbon has sp2 hybrid orbitals. These orbitals interact to make a bond. Why are these hybrid orbitals not orthogonal?arrow_forward
- Calcium cyanamide, CaNCN, is used both to kill weeds and as a fertilizer. Give the Lewis structure of the NCN2 ion and the bonded-atom lone-pair arrangement and hybridization of the carbon atom.arrow_forwardMethylcyanoacrylate is the active ingredient in super glues. Its Lewis structure is (a) How many sigma bonds are in the molecule? (b) How many pi bonds are in the molecule? (c) What is the hybridization of the carbon atom bonded to nitrogen? (d) What is the hybridization of the carbon atom bonded to oxygen? (e) What is the hybridization of the double-bonded oxygen?arrow_forwardDraw the Lewis structure for 1, 1-dimethylhydrazine [(CH3)2NNH2, a compound used as a rocket fuel]. What: is the hybridization for the two nitrogen atoms in this molecule? What orbitals overlap to form the bond between the nitrogen atoms?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Physical Chemistry
Chemistry
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Wadsworth Cengage Learning,

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co
INTRODUCTION TO MOLECULAR QUANTUM MECHANICS -Valence bond theory - 1; Author: AGK Chemistry;https://www.youtube.com/watch?v=U8kPBPqDIwM;License: Standard YouTube License, CC-BY