
a)
Interpretation:
The structural and molecular formulas for the compounds with color code for atoms as shown
Concept introduction:
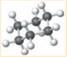
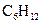 is a cyclohexane which is the chemical formula of several sugars such as fructose, glucose and galactose. It is a cyclic
is a cyclohexane which is the chemical formula of several sugars such as fructose, glucose and galactose. It is a cyclic
b)
Interpretation:
The structural and molecular formulas for the compounds with color code for atoms as shown

Concept introduction:
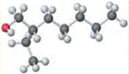
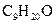 also referred to as 3-methyl-3-octanol with IUPAC name nonan-2-ol having molecular weight as 144.258 g/mol.
also referred to as 3-methyl-3-octanol with IUPAC name nonan-2-ol having molecular weight as 144.258 g/mol.
c)
Interpretation:
The structural and molecular formulas for the compounds with color code for atoms as shown
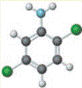
Concept introduction:
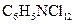 known as 2,3-dichloro aniline is soluble in ethanol, benzene and ether. It is used as an intermediate in the production of dyestuffs and also in agricultural fungicide.
known as 2,3-dichloro aniline is soluble in ethanol, benzene and ether. It is used as an intermediate in the production of dyestuffs and also in agricultural fungicide.
d)
Interpretation:
The structural and molecular formulas for the compounds with color code for atoms as shown
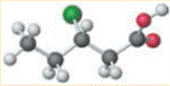
Concept introduction:
Ethyl 2-chloropropionate is a halogenated ester. It reacts with acids and releases heat along with acids and alcohols. Strong oxidizing acids result in a strong reaction that can lead to the ignition of the reaction products.
e)
Interpretation:
The structural and molecular formulas for the compounds with color code for atoms are shown.
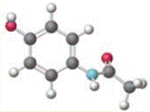
Concept introduction:
Chemical name of 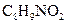 is 3-Amino-2-Hydrophenylethylketonehas. It has weak anti-inflammatory properties and is used as a common analgesic, but may cause liver, blood cell, and kidney damage.
is 3-Amino-2-Hydrophenylethylketonehas. It has weak anti-inflammatory properties and is used as a common analgesic, but may cause liver, blood cell, and kidney damage.
Want to see the full answer?
Check out a sample textbook solution
Chapter 9 Solutions
Chemistry For Changing Times (14th Edition)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





