
Chemistry For Changing Times (14th Edition)
14th Edition
ISBN: 9780321972026
Author: John W. Hill, Terry W. McCreary
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Question
Chapter 9, Problem 6CGP
Interpretation Introduction
Interpretation:
Estimation of boiling points with the use of molar mass of the following: (a) acetic acid 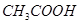 (b) acetone
(b) acetone 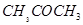 (c) ethanol
(c) ethanol 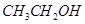 (d) hexane
(d) hexane 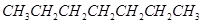 and ( e) methyl acetate
and ( e) methyl acetate 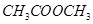 Explanation of molar mass alone being a good predictor of boiling point
Explanation of molar mass alone being a good predictor of boiling point
Concept Introduction:
Molar mass can be defined as the mass of a given subject divided by the amount of substance.
SI unit for molar mass − kg/mol. Common symbol for molar mass − M
Boiling point can be defined as the temperature of the liquid at which the liquid starts changing its state from liquid to vapour or steam. The boiling point of different liquid is different. The variation in the boiling point depends upon the surrounding environmental pressure.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Chapter 9 Solutions
Chemistry For Changing Times (14th Edition)
Ch. 9 - Prob. 1RQCh. 9 - Prob. 2RQCh. 9 - Prob. 3RQCh. 9 - Prob. 4RQCh. 9 - Prob. 5RQCh. 9 - Prob. 6RQCh. 9 - Prob. 7RQCh. 9 - Prob. 8RQCh. 9 - Prob. 9RQCh. 9 - Prob. 10RQ
Ch. 9 - Prob. 11RQCh. 9 - Prob. 12RQCh. 9 - Prob. 13RQCh. 9 - Prob. 14RQCh. 9 - Prob. 15PCh. 9 - Prob. 16PCh. 9 - Prob. 17PCh. 9 - Prob. 18PCh. 9 - Prob. 19PCh. 9 - Prob. 20PCh. 9 - Prob. 21PCh. 9 - Prob. 22PCh. 9 - Prob. 23PCh. 9 - Prob. 24PCh. 9 - Prob. 25PCh. 9 - Prob. 26PCh. 9 - Prob. 27PCh. 9 - Prob. 28PCh. 9 - Prob. 29PCh. 9 - Prob. 30PCh. 9 - Prob. 31PCh. 9 - Write the structure for (a) methyl propyl ether,...Ch. 9 - Prob. 33PCh. 9 - Prob. 34PCh. 9 - Prob. 35PCh. 9 - Prob. 36PCh. 9 - Prob. 37PCh. 9 - Prob. 38PCh. 9 - Prob. 39PCh. 9 - Prob. 40PCh. 9 - Prob. 41PCh. 9 - Prob. 42PCh. 9 - Prob. 43PCh. 9 - Prob. 44PCh. 9 - Prob. 45PCh. 9 - Prob. 46PCh. 9 - Prob. 47PCh. 9 - Prob. 48PCh. 9 - Which of the following represents a heterocyclic...Ch. 9 - Which of the following represents a heterocyclic...Ch. 9 - Prob. 51APCh. 9 - Prob. 52APCh. 9 - Prob. 53APCh. 9 - Prob. 54APCh. 9 - Prob. 55APCh. 9 - Prob. 56APCh. 9 - Prob. 57APCh. 9 - Prob. 58APCh. 9 - Prob. 59APCh. 9 - Prob. 60APCh. 9 - Refer back to Section 6.3 Describe tee type of...Ch. 9 - Prob. 62APCh. 9 - Prob. 63APCh. 9 - Prob. 64APCh. 9 - Prob. 65APCh. 9 - Prob. 66APCh. 9 - Prob. 67APCh. 9 - Prob. 68APCh. 9 - Prob. 69APCh. 9 - Prob. 70APCh. 9 - Prob. 71APCh. 9 - Prob. 72APCh. 9 - Prob. 9.1CTECh. 9 - A news feature states that herbal remedies are...Ch. 9 - Prob. 9.3CTECh. 9 - Prob. 9.4CTECh. 9 - Prob. 9.5CTECh. 9 - Prob. 9.6CTECh. 9 - Prob. 1CGPCh. 9 - Prob. 2CGPCh. 9 - Prob. 3CGPCh. 9 - Prob. 4CGPCh. 9 - Prob. 5CGPCh. 9 - Prob. 6CGPCh. 9 - Prob. 1CHQCh. 9 - Prob. 2CHQCh. 9 - Prob. 3CHQ
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Intermolecular Forces and Boiling Points; Author: Professor Dave Explains;https://www.youtube.com/watch?v=08kGgrqaZXA;License: Standard YouTube License, CC-BY