
Repeat Prob. 9–125, assuming an efficiency of 86 percent for each compressor stage and an efficiency of 90 percent for each turbine stage.
a)
The back work ratio and thermal efficiency of the gas-turbine without regenerator.
Answer to Problem 122P
The back work ratio for the ideal gas-turbine cycle without regenerator is
The thermal efficiency of the gas-turbine without regenerator is
Explanation of Solution
Draw the
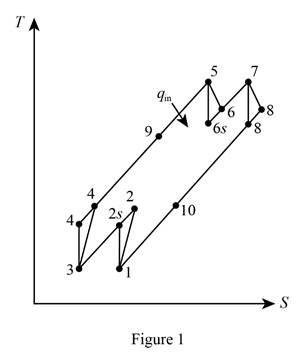
Write the pressure ratio relation for the process 1-2.
Here, relative pressure at state 1 is
Write the pressure ratio relation for the process 5-6.
Here, pressure at state 6 is
Write the expression to calculate the work input per kg to the compressors
Here, enthalpy at state 2 is
Write the expression to calculate the work done per kg by the turbines
Here, enthalpy at state 5 is
Write the expression to calculate the back work ratio
Write the expression to calculate the heat input for ideal gas-turbine cycle
Here, enthalpy at state 4 is
Write the expression to calculate the net work output per kg by the gas-turbine cycle
Write the expression to calculate the thermal efficiency of the gas-turbine cycle
Write the expression for the efficiency of the compressor
Here, the specific heat at constant pressure is
Write the expression for the efficiency of the turbine
Conclusion:
From Table A-17, “Ideal-gas properties of air”, obtain the following properties at the temperature of
Substitute 3 for
From the Table A-17, “Ideal-gas properties of air”.
Obtain the value of enthalpy on isentropic state
Write the formula of interpolation method of two variables.
Here, the variables denoted by x and y are relative pressure and enthalpy on isentropic state.
Show relative pressure and enthalpy on isentropic state values from the Table A-17.
| Relative pressure | Enthalpy |
| 4.153 | 411.12 |
| 4.158 | ? |
| 4.522 | 421.26 |
Substitute
The enthalpy on isentropic state
Substitute
From Table A-17, “Ideal-gas properties of air”, obtain the following properties at the temperature of
Substitute
From the Table A-17, “Ideal-gas properties of air” obtain the values of enthalpy on isentropic states
Substitute
Substitute
Substitute
Substitute
Thus, the back work ratio for the ideal gas-turbine cycle without regenerator is
Substitute
Substitute
Substitute
Thus, the thermal efficiency of the gas-turbine without regenerator is
b)
The thermal efficiency of the gas turbine with regenerator.
Answer to Problem 122P
The thermal efficiency of the gas turbine with regenerator is
Explanation of Solution
Write the expression to calculate the heat used for the regeneration process
Here, the effectiveness of the regenerator is
Write the expression to calculate the new heat input to the gas-turbine cycle
Write the expression to calculate the thermal efficiency of the gas-turbine with regenerator
Conclusion:
Substitute 0.75 for
Substitute
Substitute
Thus, the thermal efficiency of the gas turbine with regenerator is
Want to see more full solutions like this?
Chapter 9 Solutions
Thermodynamics: An Engineering Approach
- The turbines in steam power plants operate essentially under adiabatic conditions. A plant engineer suggests ending this practice. She proposes to run cooling water through the outer surface of the casing to cool the steam as it flows through the turbine. This way, she reasons, the entropy of the steam will decrease, the performance of the turbine will improve, and as a result the work output of the turbine will increase. How would you evaluate this proposal?arrow_forwardWhat is the Carnot cycle?arrow_forwardhow does mechanical efficiency affect net power output calculation of a gas turbine power plant. does it only affect the compressor work. does it have an effect on the net work.arrow_forward
- For a specified pressure ratio, why does multistage compression with intercooling decrease the compressor work, and multistage expansion with reheating increase the turbine work?arrow_forwardA steam turbine is equipped to bleed 6 percent of the inlet steam for feedwater heating. It is operated with 4 MPa and 350°C steam at the inlet, a bleed pressure of 800 kPa, and an exhaust pressure of 30 kPa.arrow_forwardWhat are the key factors that influence the compressible flow behavior in a gas turbine engine, and how do they impact engine performance?arrow_forward
- A turbine generates 8000 kW at 40,000 kg/hr of steam and exhaust at 10 kPa with an enthalpy of 2510 kJ/kg. If the boiler pressure is 6 Mpa, determine the isentropic efficiency.arrow_forwardWhat is the back work ratio? What are typical back work ratio values for gas-turbine engines?arrow_forwardExplain the thermodynamic processes involved in a multi-stage compression system, outlining the specific efficiency considerations at each stage, and how these processes differ from a single-stage compression system.arrow_forward
- Can the combined turbine–generator efficiency be greater than either the turbine efficiency or the generator efficiency? Explain.arrow_forwardWhat are the four processes of the Carnot cycle?arrow_forwardDefine the h-s diagram of the actual and isentropic processes of an adiabatic compressor.arrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





