
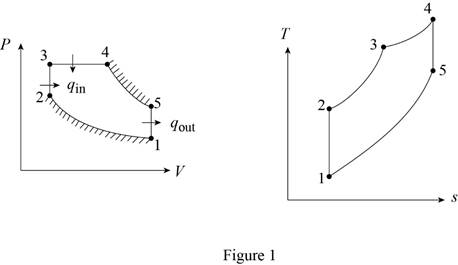
An air-standard cycle, called the dual cycle, with constant specific heats is executed in a closed piston– cylinder system and is composed of the following five processes:
1-2 Isentropic compression with a compression ratio, r = V1/V2
2-3 Constant-volume heat addition with a pressure ratio, rp = P3/P2
3-4 Constant-pressure heat addition with a volume ratio, rc V4/V3
4-5 Isentropic expansion while work is done until V5 = V1
5-1 Constant-volume heat rejection to the initial state
- (a) Sketch the P-ν and T-s diagrams for this cycle.
- (b) Obtain an expression for the cycle thermal efficiency as a function of k, r, rc, and rp.
- (c) Evaluate the limit of the efficiency as rp approaches unity, and compare your answer with the expression for the Diesel cycle efficiency.
- (d) Evaluate the limit of the efficiency as rc approaches unity, and compare your answer with the expression for the Otto cycle efficiency.
(a)
Draw the
Answer to Problem 64P
The
Explanation of Solution
Draw the
Thus, the
(b)
The expression for the back work ratio as a function of k and r.
Answer to Problem 64P
The expression for the back work ratio as a function of
Explanation of Solution
Apply first law to the closed system for processes 2-3, 3-4, and 5-1 to get the expression of
Here, heat added to the system and heat rejected from the system is
Express the cycle thermal efficiency.
Conclusion:
Process 1-2: Isentropic
Calculate the ratio of
Here, volume at states 1 and 2 is
Process 2-3: Constant volume
Calculate the expression for
Here, pressure at state 1 and 2 is
Process 3-4: Constant pressure
Calculate the expression for
Here, compression ratio is
Process 4-5: Isentropic
Calculate the expression for
Process 5-1: Constant volume
Calculate the expression for
Substitute
Calculate the ratio of
Substitute
Substitute
Substitute
Thus, the expression for the back work ratio as a function of
(c)
The limit of the efficiency as
Answer to Problem 64P
The limit of the efficiency as
Explanation of Solution
Recall the expression for the back work ratio as a function of
Thus, the limit of the efficiency as
The limit of the efficiency as
(d)
The limit of the efficiency as
Answer to Problem 64P
The limit of the efficiency as
Explanation of Solution
Recall the expression for the back work ratio as a function of
Thus, the limit of the efficiency as
The limit of the efficiency as
Want to see more full solutions like this?
Chapter 9 Solutions
Thermodynamics: An Engineering Approach
- An air-standard dual cycle has a compression ratio of 14. At the beginning of compression, p1 = 14.5 lbf/in.2, V1 = 0.5 ft3, and T1 = 50°F. The pressure doubles during the constant-volume heat addition process.For a maximum cycle temperature of 3000°R, determine:(a) the heat addition to the cycle, in Btu.(b) the net work of the cycle, in Btu.(c) the percent thermal efficiency.(d) the mean effective pressure, in lbf/in.2arrow_forwardIn a Dual cycle, the volume at the beginning of the isentropic compression is 0.85 L and the pressure is 1 bar. If the swept volume of the cycle is 0.78 L, the pressure ratio during the constant volume heat addition process is 1.9, the volume ratio during the constant pressure heat addition process is 2.1, the specific heat ratio is y=1.3 and the work done is 455 kJ/kg, the total heat added in kJ per kg of air is: Select one: o a. 705.2 • b, 949,7 o c. 521.4 o d. 1124.5arrow_forwardIn a Dual cycle, the volume at the end of the isentropic compression is 0.07 L and the pressure at the beginning of the compression stroke is 1 bar. If the swept volume of the cycle is 0.81 L, the pressure ratio during the constant volume heat addition process is 1.95, the volume ratio during the constant pressure heat addition process is 2.2, the specific heat ratio is y=1.32 and the total heat added is 896.3 kJ/kg, the work done in kJ per kg of air is: Select one: O a. 450.0 O b. 705.2 O c. 224.5 O d. 621.4arrow_forward
- An air-standard cycle with constant specific heats is executed in a closed piston–cylinder system and is composed of the following three processes: 1-2 Isentropic compression with a compression ratio r = V1/V2 2-3 Constant-pressure heat addition 3-1 Constant-volume heat rejection (a) Sketch the P-v and T-s diagrams for this cycle. (b) Obtain an expression for the back work ratio as a function of k and r.arrow_forwardAn air-standard cycle with variable specific heats is executed in a closed system with 0.0045 kg of air and consists of the following three processes: 1–2 v = Constant heat addition from 95 kPa and 17°C to 380 kPa 2–3 Isentropic expansion to 95 kPa 3–1 P = Constant heat rejection to initial state Use data from tables. Calculate the thermal efficiency % ? Hint : The answer should be a percentagearrow_forwardFor a specified compression ratio, is a diesel or gasoline engine more efficient?arrow_forward
- An air-standard dual cycle operates with a compression ratio of 18:1. At the beginning of compression the conditions are 298 K, 1 bar, and 4 L. The amount of heat added is 6.3 kJ, of which 30% is added at constant volume and the remainder at constant pressure. Determine the temperature after isentropic expansion the pressure after the constant-volume heat-addition process the temperature after the constant-pressure heat-addition process, in degrees Kelvin the thermal efficiency the temperature before the constant-pressure heat-addition process, in degrees Kelvinarrow_forwardIn a Dual cycle, the volume at the end of the isentropic compression is 0.07 L and the pressure at the beginning of the compression stroke is 1 bar. If the swept volume of the cycle is 0.81 L, the pressure ratio during the constant volume heat addition process is 1.95, the volume ratio during the constant pressure heat addition process is 2.2, the specific heat ratio is γ=1.32 and the total heat added is 896.3 kJ/kg, the work done in kJ per kg of air is:arrow_forwardThe compression ratio of an air-standard dual cycle is 10:1, it begins at 120 kPa and 300 K, and the total heat intake of the cycle has a value of 275 kJ/kg. If this heat intake at constant volume is three-fifth of the total, determine the following: V1, P2, V2, T2, T3, P3, T4, V4, T5, heat rejected, net work, volume displacement and mean effective pressure.arrow_forward
- How is the rpm (revolutions per minute) of an actual four-stroke gasoline engine related to the number of thermodynamic cycles? What would your answer be for a two-stroke engine?arrow_forwardThermodynamics Answer the following questions with complete solutions. write legibly An engine operates with air on the cycle shown with isentropic processes 1 - 2 and 3 - 4, and constant volume processes at 2 - 3 and 4 - 1. If the compression ratio (r) is 12, the minimum pressure is 200 kPa, and the maximum pressure is 10 MPa, determine the net cycle work (Wcycle) in terms of the final volume of isentropic compression process (V2). (Note: r = V1/V2 = V4/V3).arrow_forwardAn air-standard Otto cycle has a compression ratio of 9. At the beginning of compression, P1 = 100 kPa and T1 = 300 K. The heat addition per unit mass of air is 1350 kJ/kg. Determine: a) the net work, in kJ per kg of air b) the thermal efficiency of the cycle c) the mean effective pressure, in kPaarrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





