
Interpretation:
The stereoisomers formed in the reaction of acid catalysed dehydration of 3,4-dimethyl-3-hexanol and the major product has to be determined.
Concept Introduction:
Dehydration reaction:
Removal of water molecule from the reaction when the alcohol is treated with strong acid like sulfuric acid is known as dehydration reaction, for example
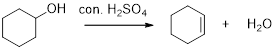
Alcohols are reacts with acids like hydrochloric acid or hydrobromic acid to yield the corresponding carbocation intermediates and then the carbocation intermediate undergoes elimination reaction to give a corresponding
The stability of carbocation is given below,
Tertiary carbocation is more stable than the secondary and primary.
Cis–trans isomerism (or) geometric isomerism or configurational isomerism:
- If the
functional groups are in the same side of the carbon chain, then it is called cis isomer. - If the functional groups are in opposite to each other in the carbon chain, then it is called Trans isomer.
- If two similar functional groups are in same side which is called as Z-isomer.
- If two similar functional groups are opposite side which is called as E-isomer.
Want to see the full answer?
Check out a sample textbook solution
Chapter 9 Solutions
Essential Organic Chemistry (3rd Edition)
- How many stereoisomers are obtained from the reaction of 3-methyl-2-butanone with each of the following organometallic reagents followed by the addition of dilute acid? Name each stereoisomer. ethyllithium methylmagnesium bromidearrow_forwardWhat products are formed when the following compounds react with CH3MgBr, followed by the addition of dilute acid? Disregard stereoisomers.arrow_forwardWhat is the most probable elimination product when 4-chlorocyclohexene is reacted with alcoholic KOH? 1,3-cyclohexadiene 1,4-cyclohexadiene Cyclohexane Cyclohexenearrow_forward
- a. Draw the major product(s) of the reaction of 1-methylcyclohexene with the following reagents, disregarding stereoisomers: 1. NBS/Δ/peroxide 2. Br2/CH2Cl2 3. HBr 4. HBr/peroxide b. For each reaction, show which stereoisomers are obtained.arrow_forwardThe reaction of 4-methylcyclohexanone with CH3MgBr followed by neutralization gives two alcohols. These two alcohols are A. enantiomers formed in equal amounts. B. diastereomers. C. constitutional isomers. D. enantiomers formed in unequal amounts.arrow_forwardWhat stereoisomers are obtained when 2-butyne undergoes each of the following reaction sequences? a. 1. H2/Lindlar catalyst 2. Br2/CH2Cl2 b. 1. Na/NH3(liq), –78 °C 2. Br2/CH2Cl2 c. 1. Cl2/CH2Cl2 2. Br2/CH2Cl2arrow_forward
- The stereochemistry of the products of reduction depends on the reagent used, as you learned in Sections 20.5 and 20.6. With this in mind, how would you convert 3,3-dimethylbutan-2-one [CH3COC(CH3)3] to: (a) racemic 3,3-dimethylbutan-2- ol [CH3CH(OH)C(CH3)3]; (b) only (R)-3,3-dimethylbutan-2-ol; (c) only (S)-3,3-dimethylbutan-2-ol?arrow_forwardIf the reaction of an alcohol with PBr3 follows an SN2 mechanism, what is the stereochemistry of the alkyl bromide formed from (R)-butan-2-ol?arrow_forwardUsing cyclohexanone as the starting material, describe how the following compounds can be synthesized:arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

