
(a)
Interpretation:
The major product has to be identified.
Concept introduction:
SN1 reaction:
The reaction of alcohols with acids like hydrochloric acid or hydrobromic which yield the corresponding carbocation intermediate, this carbocation intermediate undergoes substitution reaction which yields the corresponding substitution product.
Tertiary alcohols undergo substitution very fast than the secondary alcohols because tertiary carbocation is more stable than the secondary carbocation than the primary carbocation.
Primary alcohol is less stable therefore it won’t undergoes SN1 substitution reaction.
(b)
Interpretation:
The major product has to be identified.
Concept introduction:
SN2 reaction:
The alcohols are reaction with acids like hydrochloric acid or hydrobromic which yield the corresponding substitution product. Primary alcohol undergoes SN2 substitution reaction than secondary alcohol than tertiary alcohol because SN2 reaction is simultaneous reaction.
(c)
Interpretation:
The major product has to be identified.
Concept introduction:
Dehydration reaction:
Removal of water molecule from the reaction, the alcohol is treated with strong acid like sulfuric acid.
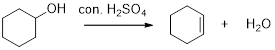
The stability of carbocation is given below,
Tertiary carbocation is more stable than the secondary and primary.
(d)
Interpretation:
The major product should be identified.
Concept introduction:
SN1 reaction:
The alcohols is reaction with acids like hydrochloric acid or hydrobromic which yield the corresponding carbocation intermediate, this carbocation intermediate undergoes substitution reaction which yields the corresponding substitution product.
Tertiary alcohols undergo substitution very fast than the secondary alcohols because tertiary carbocation is more stable than the secondary carbocation than the primary carbocation.
Primary alcohol is less stable therefore it won’t undergoes SN1 substitution reaction.
(e)
Interpretation:
The major product should be identified.
Concept introduction:
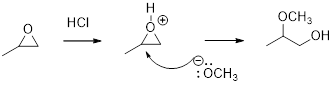
In the presence of acid catalyst, this reaction takes place through partial SN1 and partial SN2 pathway.
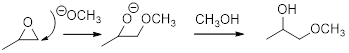
Epoxides are reactive, methoxide ion attacks the Epoxides in a less sterically hindered position which forms the alkoxide ion, and then it gets proton from alcohol which form the product.
(f)
Interpretation:
The major product should be identified.
Concept introduction:

In the presence of acid catalyst, this reaction takes place through partial SN1 and partial SN2 pathway. It is not a pure SN1 reaction because a carbocation is not formed fully and not a pure SN2 reaction because the leaving group begins to depart before the compound is attacked by the nucleophile. Epoxides are reactive; Epoxides get protonated followed by alcohol attacks to the stable carbocation and form the product.
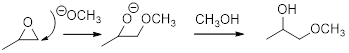
Epoxides are reactive, methoxide ion attacks the Epoxides in a less sterically hindered position which forms the alkoxide ion, and then it gets proton from alcohol which form the product. When a nucleophile attacks an unprotonated epoxide, the reaction is a pure SN2 reaction.
Note: Under acidic conditions, the nucleophile preferentially attacks the more substuituted ring carbon. Under Basic conditions, the nucleophile preferentially attacks the less substuituted ring carbon.
Want to see the full answer?
Check out a sample textbook solution
Chapter 9 Solutions
Essential Organic Chemistry (3rd Edition)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





