
Concept explainers
(a)
Interpretation:
The reason should be explained for difficulty in the synthesis of unsymmetrical ether.
Concept introduction:
Ethers:
An oxygen atom connected to two alkyl or aryl groups is called as ether. The general formula of ether is
Ethyl alcohol is reaction with acid which yields diethyl ether which is shown below.
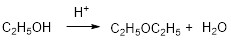
(b)
Interpretation:
The ethyl propyl ether should be synthesized.
Concept introduction:
Ethers:
An oxygen atom connected to two alkyl or aryl groups is called as ether. The general formula of ether is
Ethyl alcohol is reaction with acid which yields diethyl ether which is shown below.
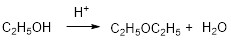
Want to see the full answer?
Check out a sample textbook solution
Chapter 9 Solutions
Essential Organic Chemistry (3rd Edition)
- Show how you might use the Williamson ether synthesis to prepare each ether. (a) (b)arrow_forwardWhat does the Ether synthesis Mechanism look like?arrow_forwardExplain why tetrahydrofuran has a higher boiling point and is much morewater soluble than furan, even though both compounds are cyclic etherscontaining four carbons.arrow_forward
- Complete these reactions. (a) (b)arrow_forwardWhich is TRUE? A. The carbonyl compound got oxidized by the Grignard reagent. B. The product as it is shown is incorrect since the alkene moieties are also expected to react. C. The methyl magnesium bromide acts as the electrophile. D. The product is not expected to undergo tautomerization since it is not an enol E. The secondary alcohol product results from the reduction of the carbonyl starting material.arrow_forwardThe cyclic hemiacetal is more stable than the open-chain form, so very little of the open-chain form is present atequilibrium. Will an aqueous solution of glucose reduce Tollens reagent and give a positive Tollens test? Explain.arrow_forward
- How Unsymmetrical ethers can be synthesized ?arrow_forwardDraw a stepwise mechanism for the attached substitution. Explain why 2-chloropyridine reacts faster than chlorobenzene in this type of reaction.arrow_forwarda. Give at least three characteristics of dichloromethane that makes it a good extracting solvent for the alkaloid.b. Why is it necessary to remove a stopper from a separatory funnel when the liquid is being drained from it through a stopcock?c. What are emulsions? Why do they form during extractions? How is the formation of an emulsion minimized? How are emulsions removed?arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

