
a)
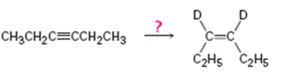
Interpretation:
How to carry out the reaction given which yields deuterium incorporated alkene as the product is to be shown.
Concept introduction:
Deuterium incorporated
To show:
How to carry out the reaction given which yields deuterium incorporated alkene as the product.
b)
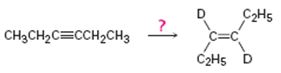
Interpretation:
How to carry out the reaction given which yields deuterium incorporated alkene as the product is to be shown.
Concept introduction:
Deuterium incorporated alkenes can be prepared from alkynes by reduction in the presence of catalysts. Use of deuterium in the presence of Lindlar catalyst yields cis alkenes with the two deuterium atoms arranged on the same side of the double bond while reduction with Li in liquid deuterated ammonia yields trans alkenes with the two deuterium atoms arranged on the opposite sides of the double bond.
To show:
How to carry out the reaction given which yields deuterium incorporated alkene as the product.
c)
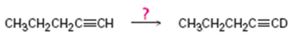
Interpretation:
How to carry out the reaction given which yields deuterium incorporated alkyne as the product is to be shown.
Concept introduction:
Deuterium incorporated alkynes can be prepared first by converting them in to alkynides by treating with NaNH2 in NH3 and then treating the alkynide obtained with D3O+.
To show:
How to carry out the reaction given which yields deuterium incorporated alkyne as the product.
d)
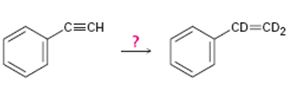
Interpretation:
How to carry out the reaction given which yields deuterium incorporated alkene as the product is to be shown.
Concept introduction:
Deuterium incorporated alkynes can be prepared first by converting them in to alkynides by treating with NaNH2 in NH3 and then treating the alkynide obtained with D3O+. The alkyne thus obtained when treated with deuterium in the presence of Lindlar catalyst yield an alkene with deuterium atom on both carbons.
To show:
How to carry out the reaction given which yields deuterium incorporated alkene as the product.
Trending nowThis is a popular solution!

Chapter 9 Solutions
Bundle: Organic Chemistry, Loose-leaf Version, 9th + LMS Integrated for OWLv2, 4 terms (24 months) Printed Access Card
- Following is an equation for iodination of toluene. This reaction does not take place. All that happens under experimental conditions for the formation of radicals is initiation to form iodine radicals, I , followed by termination to reform I2. How do you account for these observations?arrow_forwardAldehydes and ketones react with thiols to yield thioacetals just as they react with alcohols to yield acetals. Predict the product of the following reaction, and propose a mechanism:arrow_forwardBenzyl bromide is converted into benzaldehyde by heating in dimethyl sulfoxide. Propose a structure for the intermediate, and show the mechanisms of the two steps in the reaction.arrow_forward
- Predict the product for the following reaction and write a mechanism to explain how it is formed.arrow_forwardPredict the major products of treating the following compound with hot, concentrated potassium permanganate, followed by acidification with dilute HCl.(a) isopropylbenzenearrow_forwardPredict the major products of bromination of the following compounds Benzoic acid ,benzaldehyde, phenolarrow_forward
- Write the synthesis mechanism for the reaction given below and demonstrate all the steps required to obtain the product in the reaction given below, using the appropriate reagents.arrow_forwardYou are planning to carry out a reaction between propyne, CH3C≡CH and sodium amide, NaNH2. You also need to choose an appropriate solvent for carrying out the reaction. Would ethanol be suitable for this purpose? Explain your rationale clearly.arrow_forwardProvide the reagents for the short syntheses (about 3-4 steps).arrow_forward
- Propose the structures for hydrocarbons (and give the organic reaction) that give the following products on oxidative cleavage with KMnO 4 or O 3 .arrow_forwardPredict the major products of treating the following compounds with hot, concentratedpotassium permanganate, followed by acidification with dilute HCl.(a) isopropylbenzenearrow_forwardgive the products and reasonable mechanisms for the following reactions. What are the names of these two reactions?arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

