
Bundle: Organic Chemistry, Loose-leaf Version, 9th + LMS Integrated for OWLv2, 4 terms (24 months) Printed Access Card
9th Edition
ISBN: 9781305780170
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 9.SE, Problem 49AP
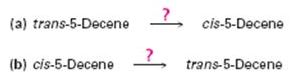
Occasionally, a chemist might need to invert the stereochemistry of an
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Draw the major product formed when HBr reacts with the following epoxide. Use wedge/dash bonds, including H's at each stereogenic center, to show the stereochemistry of the product.
The following product can be synthesized from the reaction of an alkene with Hg(OAc)2 followed by reduction with NaBH4. Draw all possible alkenes that will give the product shown
What is the major substitution product for the following reaction? Show the mechanism for the reaction.
Chapter 9 Solutions
Bundle: Organic Chemistry, Loose-leaf Version, 9th + LMS Integrated for OWLv2, 4 terms (24 months) Printed Access Card
Ch. 9.1 - Prob. 1PCh. 9.1 - Prob. 2PCh. 9.3 - What products would you expect from the following...Ch. 9.4 - Prob. 4PCh. 9.4 - Prob. 5PCh. 9.4 - Prob. 6PCh. 9.4 - Prob. 7PCh. 9.5 - Using any alkyne needed, how would you prepare the...Ch. 9.7 - The pKa of acetone, CH3COCH3, is 19.3. Which of...Ch. 9.8 - Prob. 10P
Ch. 9.8 - Prob. 11PCh. 9.9 - Show the terminal alkyne and alkyl halide from...Ch. 9.9 - Beginning with acetylene and any alkyl halide...Ch. 9.SE - Name the following alkynes, and predict the...Ch. 9.SE - From what alkyne might each of the following...Ch. 9.SE - Prob. 16VCCh. 9.SE - The following cycloalkyne is too unstable to...Ch. 9.SE - Prob. 18MPCh. 9.SE - Assuming that strong acids add to alkynes in the...Ch. 9.SE - Prob. 20MPCh. 9.SE - Prob. 21MPCh. 9.SE - Prob. 22MPCh. 9.SE - Prob. 23MPCh. 9.SE - Prob. 24MPCh. 9.SE - Reaction of acetone with D3O+ yields...Ch. 9.SE - Give IUPAC names for the following compounds:Ch. 9.SE - Draw structures corresponding to the following...Ch. 9.SE - Prob. 28APCh. 9.SE - Prob. 29APCh. 9.SE - Prob. 30APCh. 9.SE - Predict the products from reaction of l-hexyne...Ch. 9.SE - Prob. 32APCh. 9.SE - Prob. 33APCh. 9.SE - Propose structures for hydrocarbons that give the...Ch. 9.SE - Identify the reagents a-c in the following scheme:Ch. 9.SE - Prob. 36APCh. 9.SE - Prob. 37APCh. 9.SE - Prob. 38APCh. 9.SE - How would you carry out the following...Ch. 9.SE - Prob. 40APCh. 9.SE - Synthesize the following compounds using 1-butyne...Ch. 9.SE - Prob. 42APCh. 9.SE - Prob. 43APCh. 9.SE - Prob. 44APCh. 9.SE - Prob. 45APCh. 9.SE - A hydrocarbon of unknown structure has the formula...Ch. 9.SE - Compound A(C9H12) absorbed 3 equivalents of H2 on...Ch. 9.SE - Hydrocarbon A has the formula C12H8. It absorbs 8...Ch. 9.SE - Occasionally, a chemist might need to invert the...Ch. 9.SE - Prob. 50APCh. 9.SE - Prob. 51APCh. 9.SE - Prob. 52APCh. 9.SE - Prob. 53APCh. 9.SE - Prob. 54APCh. 9.SE - Prob. 55APCh. 9.SE - Prob. 56APCh. 9.SE - Prob. 57AP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Consider a reaction where cis-but-2-ene is treated with sO followed by NaHSO/H, O. Draw the structure of one product that is formed in the reaction, including correct stereochemistry.arrow_forwardDraw the products of the following reactions, indicating both regiochemistry and stereochemistry when appropriate. Use wedge and hash bonds ONLY when needed to show reaction stereochemistry. In cases where there is more than one answer, just draw one.arrow_forward1. Do propose a complete substitution mechanism of the following reactions, by looking at the given conditions determine whether it is unimolecular or bimolecular and pay attention to stereochemistry; (i) (1-bromoethyl) cyclopentane reacted with ethanol and then dimethylformamide, (ii) 2-chloro-1-cyclopropylbutane reacted with propanol.arrow_forward
- Reaction of 2° alcohol A with HCl forms three alkyl chlorides, all of which result from rearrangement of the 2° carbocation initially formed. Draw the structures of these products and a mechanism that illustrates how each is formed.arrow_forwardThe conversion of 3 alcohols into alkenes under acidic conditions involves two cationic intermediates. For each reaction, draw the complete mechanism using curved arrows.arrow_forwardOxidation of cholesterol converts the alcohol to a ketone. Under acidic or basic oxidationconditions, the C“C double bond migrates to the more stable, conjugated position. BeforeIR and NMR spectroscopy, chemists watched the UV spectrum of the reaction mixture tofollow the oxidation. Describe how the UV spectrum of the conjugated product, cholest-4-en-3-one, differs from that of cholesterolarrow_forward
- 2- Mention the product of a nucleophilic substitution reaction of (S)-2-bromohexane with acetate ion, CH3CO2- Assume that inversion of configuration occurs, and show the chemistry of both the reactant and product. 3- Draw the mechanism of the SN2 Reaction. Show the correct directions of the arrows, and all reagents. 4- Draw the mechanism of the SN1 Reaction. Show the correct directions of the arrows, and all reagents. 5- Draw the mechanism of the E2 Reaction with an Alkyl Halide. Show the correct directions of the arrows, and all reagents. 6- Draw the mechanism of the E1 Reaction with an Alkyl Halide. Show the correct directions of the arrows, and all reagents. 7- Mention the product formed in an SN2 reaction between 1-bromobutane and NaI. 8- Rank the following compounds in order of their expected reactivity toward SN2 reaction: CH3Br, CH3OTos, (CH3)2CHCl. 9- Explain Grignard Reagents in details with one example. 10- Mention how a halogen substituent can be replaced by a deuterium atom…arrow_forwardDraw the substitution product formed (including stereochemistry) when (R)-hexan-2-ol is treated with each series of reagents: (a) NaH, followed by CH3I; (b) TsCl and pyridine, followed by NaOCH3; (c) PBr3, followed by NaOCH3. Which two routes produce identical products?arrow_forwardDraw the products formed when the following alkynes are treated with each set of reagents: [1] H2O, H2SO4, HgSO4; or [2] R2BH followed by H2O2, −OH.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you

 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Alcohols, Ethers, and Epoxides: Crash Course Organic Chemistry #24; Author: Crash Course;https://www.youtube.com/watch?v=j04zMFwDeDU;License: Standard YouTube License, CC-BY