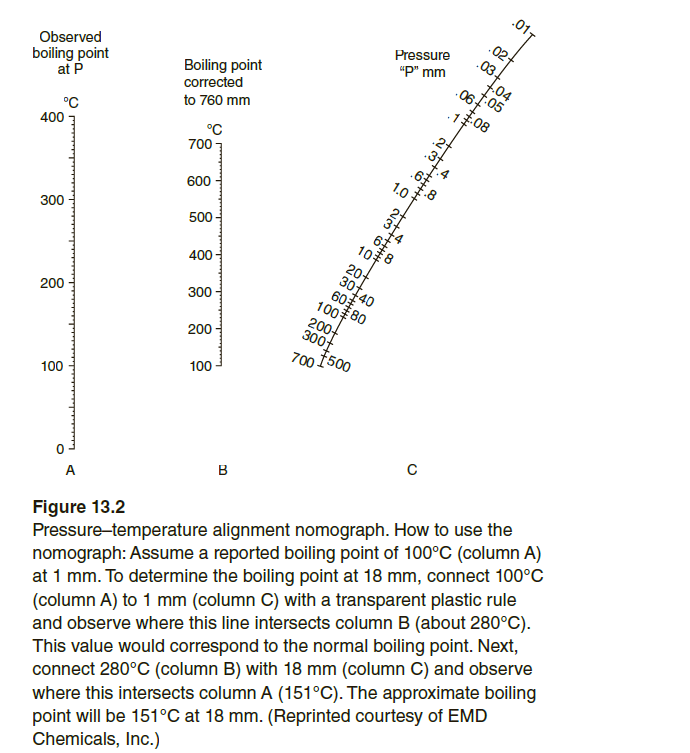
.011 .02- Pressure "P" mm Observed boiling point at P Boiling point corrected to 760 mm °C °C 400 700 1.0 600 300 6 500 - 20 30140 60 100 80 200- 300f 400 - 300 200 200 700 1500 100 100 в A Figure 13.2 Pressure-temperature alignment nomograph. How to use the nomograph: Assume a reported boiling point of 100°C (column A) at 1 mm. To determine the boiling point at 18 mm, connect 100°C (column A) to 1 mm (column C) with a transparent plastic rule and observe where this line intersects column B (about 280°C). This value would correspond to the normal boiling point. Next, connect 280°C (column B) with 18 mm (column C) and observe where this intersects column A (151°C). The approximate boiling point will be 151°C at 18 mm. (Reprinted courtesy of EMD Chemicals, Inc.) 4. O 88 +++
.011 .02- Pressure "P" mm Observed boiling point at P Boiling point corrected to 760 mm °C °C 400 700 1.0 600 300 6 500 - 20 30140 60 100 80 200- 300f 400 - 300 200 200 700 1500 100 100 в A Figure 13.2 Pressure-temperature alignment nomograph. How to use the nomograph: Assume a reported boiling point of 100°C (column A) at 1 mm. To determine the boiling point at 18 mm, connect 100°C (column A) to 1 mm (column C) with a transparent plastic rule and observe where this line intersects column B (about 280°C). This value would correspond to the normal boiling point. Next, connect 280°C (column B) with 18 mm (column C) and observe where this intersects column A (151°C). The approximate boiling point will be 151°C at 18 mm. (Reprinted courtesy of EMD Chemicals, Inc.) 4. O 88 +++
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter7: Equilibria In Multiple-component Systems
Section: Chapter Questions
Problem 7.64E
Related questions
Question
Using the pressure–temperature alignment chart in Figure 13.2, answer the
following questions.
a. What is the normal boiling point (at 760 mm Hg) for a compound that boils
at 150°C at 10 mm Hg pressure?
b. At what temperature would the compound in (a) boil if the pressure were
40 mm Hg?
c. A compound was distilled at atmospheric pressure and had a boiling point
of 285°C. What would be the approximate boiling range for this compound
at 15 mm Hg?
Transcribed Image Text:.011
.02-
Pressure
"P" mm
Observed
boiling point
at P
Boiling point
corrected
to 760 mm
°C
°C
400
700
1.0
600
300
6
500 -
20
30140
60
100 80
200-
300f
400 -
300
200
200
700 1500
100
100
в
A
Figure 13.2
Pressure-temperature alignment nomograph. How to use the
nomograph: Assume a reported boiling point of 100°C (column A)
at 1 mm. To determine the boiling point at 18 mm, connect 100°C
(column A) to 1 mm (column C) with a transparent plastic rule
and observe where this line intersects column B (about 280°C).
This value would correspond to the normal boiling point. Next,
connect 280°C (column B) with 18 mm (column C) and observe
where this intersects column A (151°C). The approximate boiling
point will be 151°C at 18 mm. (Reprinted courtesy of EMD
Chemicals, Inc.)
4.
O 88
+++
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning