2. The following tyrosine-phosphorylated polypeptides were tested as substrates of protein tyrosine phosphatase, an important enzyme in cellular signal transduction. The pTyr in bold signifies the tyrosine residue that is phosphorylated. The binding affinities of the polypeptide substrates to the enzyme were found to follow the order: (a) > (c) × (d) >> (b). (a) Asp-Ala-Asp-Glu-pTyr-Leu-lle-Pro-Gln-GIn-Gly (b) Pro-Val-Pro-Glu-pTyr-lle-Asn-Ala-Ser-Val (c) Asp-Ala-Glu-Glu-pTyr-Leu-Val-Pro-Gln-Gln-Gly (d) Asp-Asn-Leu-Tyr-pTyr-Trp-Asp-Glu-Asn-Ser-Ser In addition to the presence of the phosphorylated tyrosine residue, what amino acid a) properties of the polypeptides accounts for the relative order of binding affinities to the enzyme?
Gene Interactions
When the expression of a single trait is influenced by two or more different non-allelic genes, it is termed as genetic interaction. According to Mendel's law of inheritance, each gene functions in its own way and does not depend on the function of another gene, i.e., a single gene controls each of seven characteristics considered, but the complex contribution of many different genes determine many traits of an organism.
Gene Expression
Gene expression is a process by which the instructions present in deoxyribonucleic acid (DNA) are converted into useful molecules such as proteins, and functional messenger ribonucleic (mRNA) molecules in the case of non-protein-coding genes.
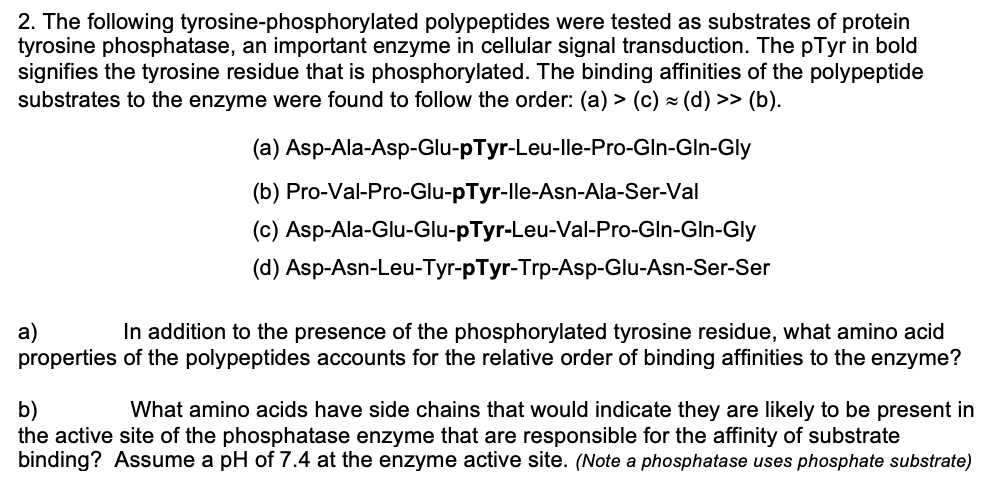
Enzymes are proteins that catalyze various reactions inside the cellular environment. Enzyme binds to the substrate through their binding site and catalyzes the reactions. Tyrosine phosphatase removes the phosphate group from tyrosine residues present in peptides.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps


