
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
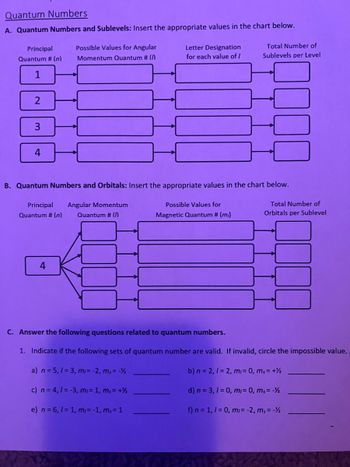
Transcribed Image Text:Quantum Numbers
A. Quantum Numbers and Sublevels: Insert the appropriate values in the chart below.
Principal
Quantum # (n)
1
2
3
4
Possible Values for Angular
Momentum Quantum # (/
4
Letter Designation
for each value of /
Total Number of
Sublevels per Level
B. Quantum Numbers and Orbitals: Insert the appropriate values in the chart below.
Principal Angular Momentum
Quantum # (n) Quantum #(D)
Possible Values for
Magnetic Quantum # (m)
Total Number of
Orbitals per Sublevel
C. Answer the following questions related to quantum numbers.
1. Indicate if the following sets of quantum number are valid. If invalid, circle the impossible value.
a) n = 5,1 = 3, m/= -2, ms = -1/2
b) n = 2, 1 = 2, m/= 0, ms = + ½
c) n = 4, 1= -3, mi = 1, ms = + ½
d) n = 3, 1 = 0, mi= 0, ms = -½
e) n = 6, 1 = 1, mi = -1, ms = 1
f) n = 1, 1 = 0, m₁ = -2, ms = -½
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the smallest possible value of the principal quantum number n for a p electron? TOCAMLAGI Jobinel 11 = Continue 0 1-95 S/US-1 S/... Closed road X 3 Q Search S 2023 McGraw Hill LLCarrow_forward101 Chem101 b My Questions | bartleby x + -> app.101edu.co M Apps G M Gmail YouTube Maps a AMAZON Translate O Gflights Case Status Onlin... b Homework Help a... C Get Homework He... > KATAPULK CUBA 23 Agencia Supermar.. Reading List Question 3 of 22 Submit Which of the following is not permitted as a plausible orbital designation according to quantum theory. A) 4f B) 7s C) Зр D) 3f +arrow_forwardUB8isprv3D&drc%3D1&qi%3D660687&tcfql3D SSSTIREE 101 Chem101 I Balance Chemical E... Z Score Calculator.. My Application t 3 Vocabulary Matching Assignment ngth: 2:00:00 Starline Chardonette: Attempt 1 The distance between 80 3 adjacent crests of a wave The range of the wavelengths of all possible electromagnetic radiation For waves, the number of cycles (or complete wavelengths) that pass through a stationary point in one second An integer that specifies the orientation of an orbital A model that explains the behavior of absolutely small particles such as electrons and photons The probability (per unit volume) of finding the electron at a point in space as expressed by a three-dimensional plot of the wave function squared 1. angular mornentum quantum A form of energy number embodied in oscillating electric and magnetic fields 2. deBroglie relation 3. electromagnetic radiation The range of wavelengths emitted by a particular 4. electromagnetic spectrum element; used to identify the…arrow_forward
- What is the number of angular nodes in each case? a.) 1s (angular nodes) b.) 3s (angular nodes) c.) 3d (angular nodes)arrow_forwardThe solution of the Schrödinger wave equation for the hydrogen atom results in a set of functions (orbitals) that describe the behavior of the electron. Each function is characterized by three quantum numbers: n, l, and ml. If the value of n = 1... The quantum number l can have values from ___to ___.... The total number of orbitals possible at the n = 1 energy level is ____.If the value of l = 0... The quantum number ml can have values from ___ to ____.... The total number of orbitals possible at the l = 0 sublevel is ____.arrow_forwardPls help ASAP. Pls do both of the asked parts.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY