CHEM3322 EXPERIMENT 3: Extraction Name: ID: Q1. Give three criteria for a good extraction solvent. Q2. Write a balanced chemical equation for the acid-base reactions involved in this experiment. Q3. Which is the bottom phase in a water/methylene chloride (d=1.325) system? Briefly explain your predictions
CHEM3322 EXPERIMENT 3: Extraction Name: ID: Q1. Give three criteria for a good extraction solvent. Q2. Write a balanced chemical equation for the acid-base reactions involved in this experiment. Q3. Which is the bottom phase in a water/methylene chloride (d=1.325) system? Briefly explain your predictions
Chapter11: Isolation Of Caffeine From Tea Le
Section: Chapter Questions
Problem 1Q
Related questions
Question
EXTRACTION SEPARATION OF BENZOIC ACID FROM A MIXTURE OF BENZOIC ACID AND NAPHTHALENE
Transcribed Image Text:12 of 78
EXPERIMENTAL PROCEDURE:
1. Weigh to two decimal places, about 2 g of the mixture of benzoic acid and naphthalene
and transfer it to a 100 mL conical flask. Record the mass, accurately, in the results
report.
2. Add about 40 mL of ethyl acetate (an organic solvent) into the conical flask and shake
it gently until all the solid has dissolved.
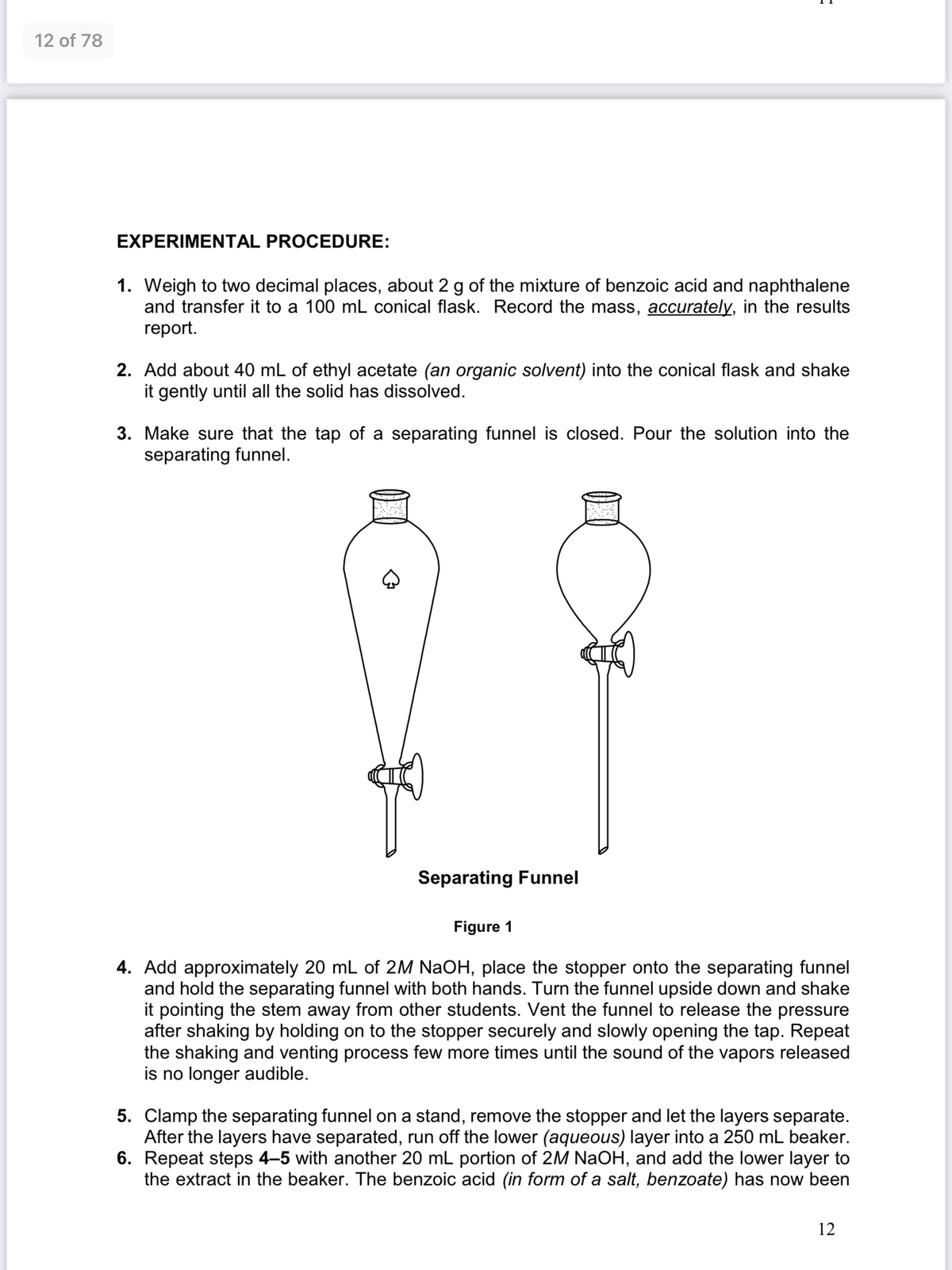
3. Make sure that the tap of a separating funnel is closed. Pour the solution into the
separating funnel.
Separating Funnel
Figure 1
4. Add approximately 20 mL of 2M NaOH, place the stopper onto the separating funnel
and hold the separating funnel with both hands. Turn the funnel upside down and shake
it pointing the stem away from other students. Vent the funnel to release the pressure
after shaking by holding on to the stopper securely and slowly opening the tap. Repeat
the shaking and venting process few more times until the sound of the vapors released
is no longer audible.
5. Clamp the separating funnel on a stand, remove the stopper and let the layers separate.
After the layers have separated, run off the lower (aqueous) layer into a 250 mL beaker.
6. Repeat steps 4-5 with another 20 mL portion of 2M NaOH, and add the lower layer to
the extract in the beaker. The benzoic acid (in form of a salt, benzoate) has now been
12

Transcribed Image Text:CHEM3322
EXPERIMENT 3: Extraction
Name:
ID:
Q1. Give three criteria for a good extraction solvent.
Q2. Write a balanced chemical equation for the acid-base reactions involved in this
experiment.
Q3. Which is the bottom phase in a water/methylene chloride (d=1.325) system? Briefly
explain your predictions
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT