density of nickel (d 8.91 g/cm') to determine the volume in cubic centimeters of a sample that has a mass of 37.0 pounds. d 453.6 grams) cm eck & Submit Answer Show Approach
density of nickel (d 8.91 g/cm') to determine the volume in cubic centimeters of a sample that has a mass of 37.0 pounds. d 453.6 grams) cm eck & Submit Answer Show Approach
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter3: Measurement And Chemical Calculations
Section: Chapter Questions
Problem 128E: In Active Example 3-29 you calculated that you would have to work six weeks to earn enough money to...
Related questions
Question
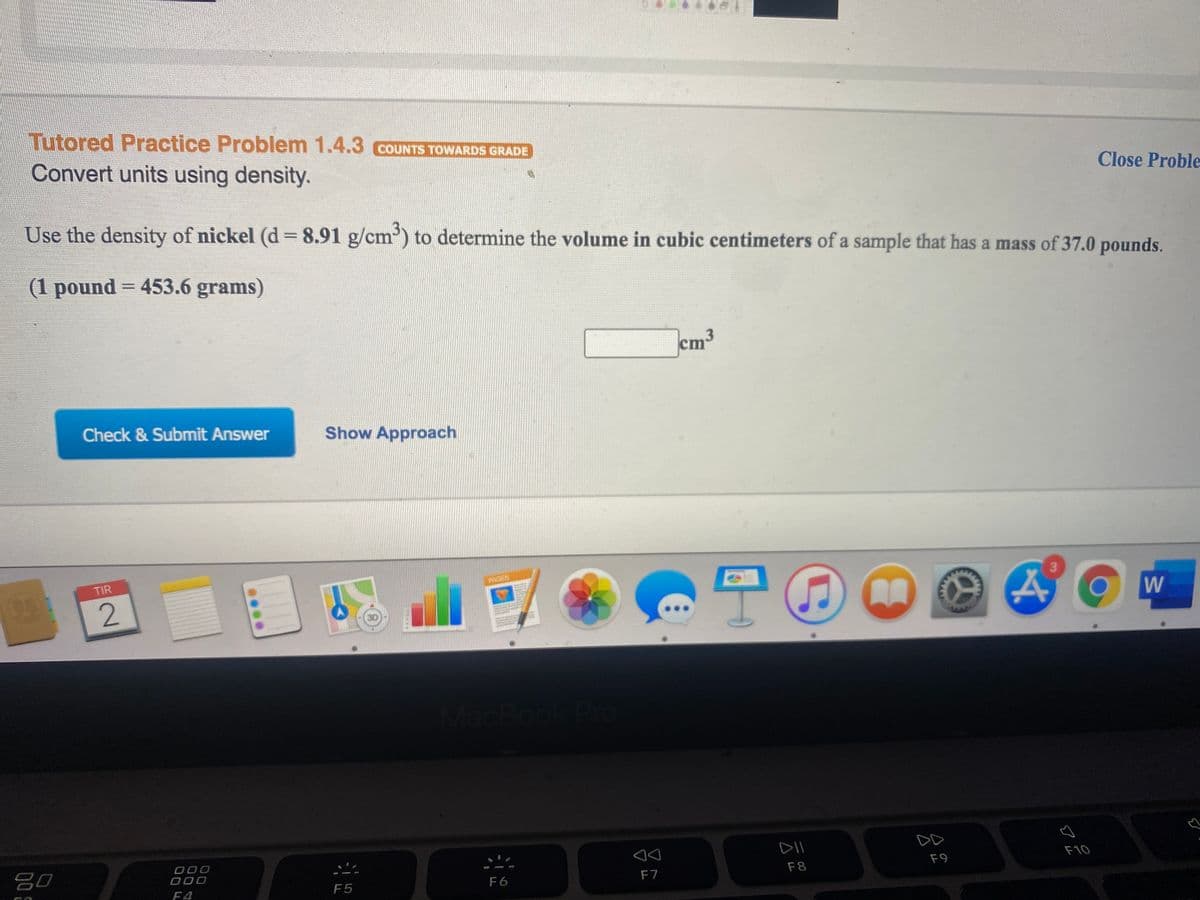
Transcribed Image Text:Tutored Practice Problem 1.4.3 COUNTS TOWARDS GRADE
Convert units using density.
Close Proble
Use the density of nickel (d =8.91 g/cm') to determine the volume in cubic centimeters of a sample that has a mass of 37.0 pounds.
(1 pound = 453.6 grams)
%3D
cm
ст
Check & Submit Answer
Show Approach
TIR
PAGES
3
W
MacBook Pro
000
000
F10
80
F9
F8
F6
F7
F4
F5
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning


Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning
