P18C.2 The rates of thermal decomposition of a variety of cis- and trans- azoalkanes have been measured over a range of temperatures in order to settle a controversy concerning the mechanism of the reaction. In ethanol an unstable cis-azoalkane decomposed at a rate that was followed by observing the N, evolution, and this led to the rate constants listed below (P.S. Engel and D.J. Bishop, J. Amer. Chem. Soc. 97, 6754 (1975)). Calculate the enthalpy, entropy, energy, and Gibbs energy of activation at -20 °C. -24.82 -20.73 -17.02 -13.00 -8.95 10ʻ x k/5" 14.3 1.22 2.31 4.39 8.50
P18C.2 The rates of thermal decomposition of a variety of cis- and trans- azoalkanes have been measured over a range of temperatures in order to settle a controversy concerning the mechanism of the reaction. In ethanol an unstable cis-azoalkane decomposed at a rate that was followed by observing the N, evolution, and this led to the rate constants listed below (P.S. Engel and D.J. Bishop, J. Amer. Chem. Soc. 97, 6754 (1975)). Calculate the enthalpy, entropy, energy, and Gibbs energy of activation at -20 °C. -24.82 -20.73 -17.02 -13.00 -8.95 10ʻ x k/5" 14.3 1.22 2.31 4.39 8.50
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter11: Chemical Kinetics: Rates Of Reactions
Section: Chapter Questions
Problem 83QRT
Related questions
Question
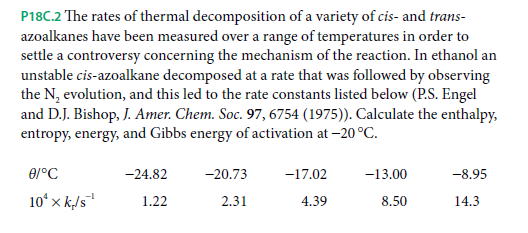
Transcribed Image Text:P18C.2 The rates of thermal decomposition of a variety of cis- and trans-
azoalkanes have been measured over a range of temperatures in order to
settle a controversy concerning the mechanism of the reaction. In ethanol an
unstable cis-azoalkane decomposed at a rate that was followed by observing
the N, evolution, and this led to the rate constants listed below (P.S. Engel
and D.J. Bishop, J. Amer. Chem. Soc. 97, 6754 (1975)). Calculate the enthalpy,
entropy, energy, and Gibbs energy of activation at -20 °C.
-24.82
-20.73
-17.02
-13.00
-8.95
10ʻ x k/5"
14.3
1.22
2.31
4.39
8.50
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 3 images

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,