Parameter Value 1. Benzoic acid sample weight, g 0.1702 2. Water volume used, mL 10.0 3. Methylene chloride volume used, mL 5.0 4. Number of extractions 3 5. Erlenmeyer flask weight, g 93.6201 6. Erlenmeyer flask + recovered sample weight, g 93.7841
Parameter Value 1. Benzoic acid sample weight, g 0.1702 2. Water volume used, mL 10.0 3. Methylene chloride volume used, mL 5.0 4. Number of extractions 3 5. Erlenmeyer flask weight, g 93.6201 6. Erlenmeyer flask + recovered sample weight, g 93.7841
Chapter14: Chromatography
Section: Chapter Questions
Problem 9P
Related questions
Question
100%
From the following data (given table in picture 1), solve using this equation (picture 2)
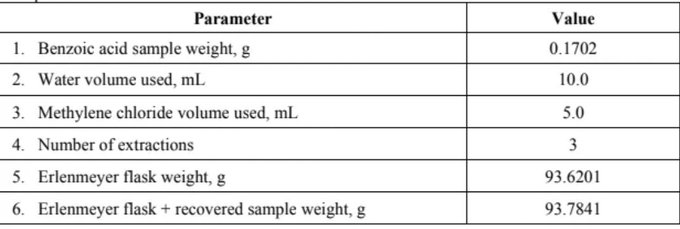
Transcribed Image Text:Parameter
Value
1. Benzoic acid sample weight, g
0.1702
2. Water volume used, mL
10.0
3. Methylene chloride volume used, mL
5.0
4. Number of extractions
3
5. Erlenmeyer flask weight, g
93.6201
6. Erlenmeyer flask + recovered sample weight, g
93.7841

Transcribed Image Text:Notes: k= (solubility in H2O/solubility in DCM)= 6.0 (at 25°C)
V2
V2 +V,k)
(Final mass of solute)H20
(Initial mass of solute)H,0
Where: V= volume of organic solvent used per extraction
V2= original volume of water
n= number of extractions
k= distribution coefficient
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

