The following equation describes the dependence of the rate constant (k) with the temperature in dynamic NMR. -AG*. k = - exp h RT. where k and h are physical constants, R is the ideal gas constant, T. is the coalescence temperature and AG* is the activation energy? The following table shows the units of some of these parameters. Parameters Units k s-l Tc K h Js JK-' mol· J mol- R AG* Does k increase with AG*? Explain your answer.
The following equation describes the dependence of the rate constant (k) with the temperature in dynamic NMR. -AG*. k = - exp h RT. where k and h are physical constants, R is the ideal gas constant, T. is the coalescence temperature and AG* is the activation energy? The following table shows the units of some of these parameters. Parameters Units k s-l Tc K h Js JK-' mol· J mol- R AG* Does k increase with AG*? Explain your answer.
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter32: Radiochemical Methods
Section: Chapter Questions
Problem 32.12QAP
Related questions
Question
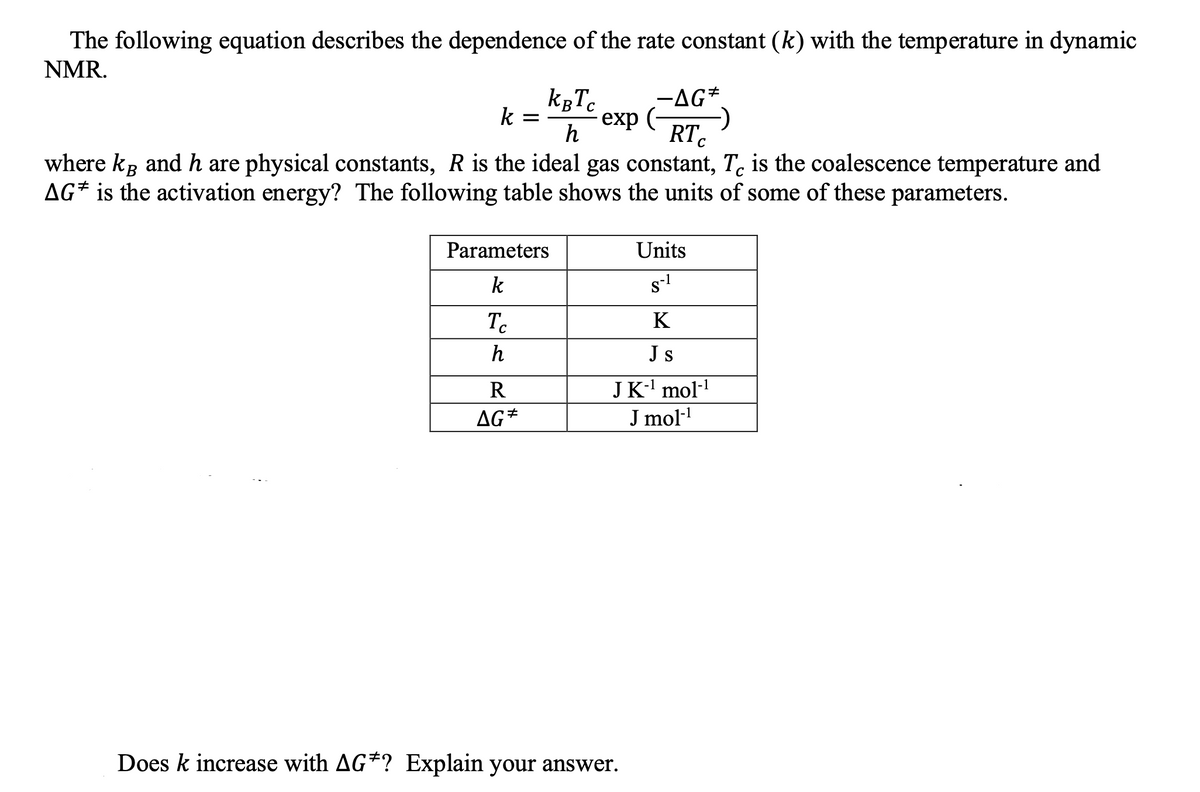
Transcribed Image Text:The following equation describes the dependence of the rate constant (k) with the temperature in dynamic
NMR.
kgTc
-AG*.
exp (-
RT.
k =
C
where kg and h are physical constants, R is the ideal gas constant, T. is the coalescence temperature and
AG* is the activation energy? The following table shows the units of some of these parameters.
Parameters
Units
k
s-1
To
K
h
Js
R
JK-' mol-!
AG*
J mol-
Does k increase with AG*? Explain your answer.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning