Volume delivered by 10 mL pipet (show calculations) mL Mean volume delivered by 10 mL pipet (show calculations) Trial 3 Trial 1 Trial 2 Individual deviations from the mean Average deviation from the mean (show calculations) mL Volume delivered by your 10 mL pipet mL + mL
Volume delivered by 10 mL pipet (show calculations) mL Mean volume delivered by 10 mL pipet (show calculations) Trial 3 Trial 1 Trial 2 Individual deviations from the mean Average deviation from the mean (show calculations) mL Volume delivered by your 10 mL pipet mL + mL
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
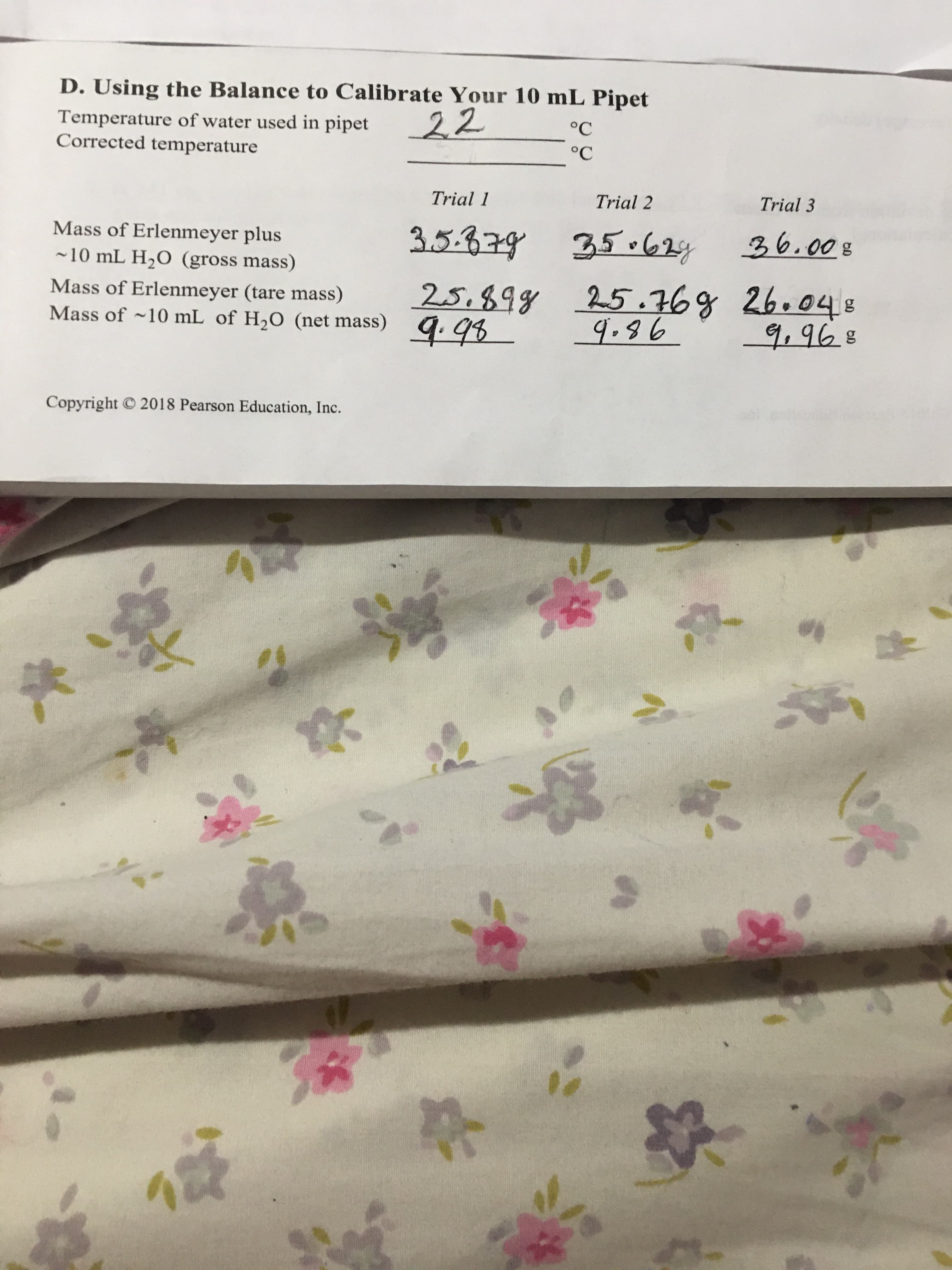
Transcribed Image Text:D. Using the Balance to Calibrate Your 10 mL Pipet
Temperature of water used in pipet
Corrected temperature
22
°C
°C
Trial 1
Trial 2
Trial 3
Mass of Erlenmeyer plus
-10 mL H,O (gross mass)
3209を
Mass of Erlenmeyer (tare mass)
Mass of ~10 mL of H,0 (net mass)
25.89g 25.76g 26.048
9.86
Copyright © 2018 Pearson Education, Inc.

Transcribed Image Text:14 Report Sheet
Basic Laboratory Techniques
Volume delivered by 10 mL
pipet (show calculations)
Mean volume delivered by 10 mL pipet (show calculations)
Trial 3
Trial 1
Trial 2
Individual deviations from the mean
Average deviation from the mean (show calculations)
Volume delivered by your 10 mL pipet
am oi eau ot iserw ert ,bnuong or i pnigpib 1ot ecd orl lo yievooatb enfops eiceY
ske
a1ebnow pniseme ee blard lis eutsieggs ns vd eoboo bnea of vilide ert evom
liso et enigeraiton bib emit erl is bevil orw Jeom videdon9 nevooaib to
veidoe of yoolonbel roue to tened ert ons vebot to anetuomoo er no
sd jerit hoimco erit bne vsbo to einemeonevbe ent tuods swe ni ed vilsutos yem
viinstiog ni laom vsbol asonevDs Isoipolonbet yoine ew es doum 2s lau, be
emit larii is elgosg to 2evil ert bevoigmi 2eonsvbe
to becbje
evbe eas
uCy lieseb ni aaib bne noitenipami nucy eeU Seten mo op
biswot paivom bns 020S ni eenevobeib wen 1olbns yrolonnioerjuod
diw.doengensg eno batete a0 bluoria eenc0
esonainer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY