
Concept explainers
(a)
Interpretation:
The Lewis structure of the
Concept introduction:
Lewis structure of a molecule or ion is drawn considering only the valence electrons of the atoms. In case of ions, for each negative charge an electron is added while for each positive charge an electron is subtracted on the ion. Each bond shows a shared pair of electrons. Single, double, and triple bonds are represented by one, two, and three lines, respectively, connecting the two atoms. Non-bonding electrons are shown as dots, generally as lone pairs. Atoms in a Lewis structure should not exceed their valency. The atoms from the 2nd row must not exceed an octet of valence electrons. Atoms from the 3rd row onward may exceed an octet by up to four electrons if they are central atoms.
The formal charge (FC) on an atom is determined as the difference between its group number and the actual number of electrons it possesses in the molecule or ion. The formula to calculate formal charge is as below.
Answer to Problem 1.50P
The Lewis structure of the
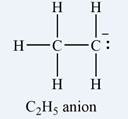
The atom that bears a non-zero formal charge is the carbon atom on the right, bonded to only two hydrogens.
Explanation of Solution
The total of valence electrons based on the formula
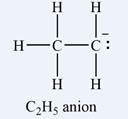
The formal charge on each hydrogen atom is zero:
The formal charge on the first carbon (on the left) is zero:
The formal charge on the second carbon is
Therefore, it is the carbon atom on the right, bearing only two hydrogen atoms, that has a non-zero formal charge.
The formal charge on an atom is the difference in its group number and the actual number of electrons it possesses in the molecule or ion.
(b)
Interpretation:
The Lewis structure of the
Concept introduction:
Lewis structure of a molecule or ion is drawn considering only the valence electrons of the atoms. Each bond shows a shared pair of electrons. Single, double, and triple bonds are represented by one, two, and three lines, respectively, connecting the two atoms. Non-bonding electrons are shown as dots, generally as lone pairs. Atoms in a Lewis structure should not exceed their valency. The atoms from the 2nd row must not exceed an octet of valence electrons. Atoms from the 3rd row onward may exceed an octet by up to four electrons if they are central atoms.
The formal charge on an atom is determined as the difference between its group number and the actual number of electrons it possesses in the molecule or ion. The formula to calculate formal charge is as below.
Answer to Problem 1.50P
The Lewis structure of the
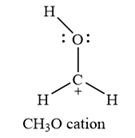
The carbon atom is the one that has a non-zero formal charge.
Explanation of Solution
Carbon is the central atom in this cation, with two hydrogen atoms and the oxygen atom bonded to it. The third hydrogen atom is bonded to the oxygen as shown below:
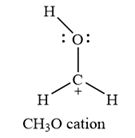
The formal charges on the atoms are calculated as below:
Hydrogen atoms:
Carbon atom:
Oxygen atom:
An alternate structure can be drawn with all three hydrogen atoms and the oxygen atom bonded to the central carbon. However, this will put the positive charge on a highly electronegative oxygen, making it unstable. Therefore, the above mentioned structure is the most likely structure.
Therefore, the carbon atom has a non-zero formal charge.
The formal charge on an atom is the difference in its group number and the actual number of electrons it possesses in the molecule or ion.
(c)
Interpretation:
The Lewis structure of the
Concept introduction:
Lewis structure of a molecule or ion is drawn considering only the valence electrons of the atoms. Each bond shows a shared pair of electrons. Single, double, and triple bonds are represented by one, two, and three lines, respectively, connecting the two atoms. Non-bonding electrons are shown as dots, generally as lone pairs. Atoms in a Lewis structure should not exceed their valency. The atoms from the 2nd row must not exceed an octet of valence electrons. Atoms from the 3rd row onward may exceed an octet by up to four electrons if they are central atoms.
The formal charge on an atom is determined as the difference between its group number and the actual number of electrons it possesses in the molecule or ion. The formula to calculate formal charge is as below.
Answer to Problem 1.50P
The Lewis structure of the
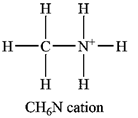
The atom with a non-zero formal charge is the nitrogen.
Explanation of Solution
Carbon usually forms four bonds; therefore, it is a central atom. Three of the hydrogen atoms and the nitrogen atom must be bonded to it. The nitrogen atom in turn is also a central atom, with the remaining three hydrogen atoms bonded to it. Normally nitrogen forms only three bonds, but it can use its lone pair to form another bond, generally to a hydrogen ion. Therefore, the Lewis structure of the cation must be

The formal charges on the atoms are calculated as follows:
Hydrogen atoms:
Carbon atom:
Nitrogen atom:
Therefore, it is the nitrogen atom that has a non-zero formal charge.
The formal charge on an atom is the difference in its group number and the actual number of electrons it possesses in the molecule or ion.
(d)
Interpretation:
The Lewis structure of the
Concept introduction:
Lewis structure of a molecule or ion is drawn considering only the valence electrons of the atoms. Each bond shows a shared pair of electrons. Single, double, and triple bonds are represented by one, two, and three lines, respectively, connecting the two atoms. Non-bonding electrons are shown as dots, generally as lone pairs. Atoms in a Lewis structure should not exceed their valency. The atoms from the 2nd row must not exceed an octet of valence electrons. Atoms from the 3rd row onward may exceed an octet by up to four electrons if they are central atoms.
The formal charge on an atom is determined as the difference between its group number and the actual number of electrons it possesses in the molecule or ion. The formula to calculate formal charge is as below.
Answer to Problem 1.50P
The Lewis structure of
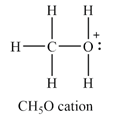
The atom with non-zero formal charge is the oxygen atom.
Explanation of Solution
The

The formal charges on the atoms are calculated as follows:
Hydrogen atoms:
Carbon atom:
Oxygen atom:
Therefore, the oxygen has a non-zero formal charge.
The formal charge on an atom is the difference in its group number and the actual number of electrons it possesses in the molecule or ion.
(e)
Interpretation:
The Lewis structure of the
Concept introduction:
Lewis structure of a molecule or ion is drawn considering only the valence electrons of the atoms. Each bond shows a shared pair of electrons. Single, double, and triple bonds are represented by one, two, and three lines, respectively, connecting the two atoms. Non-bonding electrons are shown as dots, generally as lone pairs. Atoms in a Lewis structure should not exceed their valency. The atoms from the 2nd row must not exceed an octet of valence electrons. Atoms from the 3rd row onward may exceed an octet by up to four electrons if they are central atoms.
The formal charge on an atom is determined as the difference between its group number and the actual number of electrons it possesses in the molecule or ion. The formula to calculate formal charge is as below.
Answer to Problem 1.50P
The Lewis structure of the
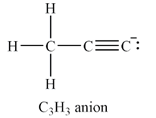
The carbon atom at the right end of the chain has a non-zero formal charge.
Explanation of Solution
The total of the valence electrons for this anion is
The carbon atoms have capacity to form up to four bonds. If all three hydrogen atoms are on the same carbon, this carbon must be at the end of a chain of three carbon atoms. The other two carbon atoms must be triply bonded to each other in order to complete their octets. The carbon atom at the other end of the chain must have a lone pair so that all the valence electrons are accounted for.
The Lewis structure of the

The formal charges on the atoms are calculated as follows:
Hydrogen atoms:
Carbon atom on the left and in the center of the chain:
Carbon atom at the right end of the chain:
Therefore, it is the carbon atom at the right end of the chain that has a non-zero formal charge.
The formal charge on an atom is the difference in its group number and the actual number of electrons it possesses in the molecule or ion.
Want to see more full solutions like this?
Chapter 1 Solutions
ORGANIC CHEMISTRY E-BOOK W/SMARTWORK5
- 3-109 Until several years ago, the two chlorofluorocarbons (CFCs) most widely used as heat transfer media in refrigeration systems were Freon-li (trichloro fluoromethane, CC13F) and Freon-12 (dichiorodi fluoromethane, CCl2F2). Draw a three-dimensional representation of each molecule and indicate the Direction of it.s polarity.arrow_forwardWhat is the formal charge of each atom (from left to right), neglecting the hydrogens, in the following resonance structure of CH2N2? Select one: a. 0, +1, −1 b. 0, −1, +1 c. −1, −1, +1 d. −1, +1, 0 e. 0, 0, 0arrow_forwardWRITE THE COMPLETE COMPUTATION of the FORMAL CHARGE of each atom using the formula: FC = V - N - B/2 a. Ethanol b. Chlorate ionarrow_forward
- Consider one resonance structure for the azide ion, N3-1 : What is the formal charge on each nitrogen atom in this resonance structure? Enter your answer as a number preceded by the appropriate charge (+ or -), if applicable. If the formal charge is zero, enter 0 . formal charge of left N [a] formal charge of middle N [b] formal charge of right N [c]arrow_forwardIn which of the structures shown below does each atom have a formal charge of zero?arrow_forwardDraw one of the possible resonance structures for the chlorate ion (ClO3−). Be sure to include all lone pair electrons and nonzero formal charges.arrow_forward
- What is the formal charge of the carbon atom in the structure below?arrow_forwardFor each ion below, draw all reasonable resonance structures (linked by resonance arrows “↔”). Include curved arrows that indicate the movement of electrons between each resonance structure. Assign non-zero formal charges to each atom for each resonance structure. (a.) NO3– (nitrate) (b.) CH3COO– (acetate) (c.) N3– (azide) (d.) NCO– (isocyanate)arrow_forward#1: What is the formal charge of bromine in the structure of bromate, BrO3-? a) 0 b) +1 c) +2 d) -1 #2: Carbonate (CO32-) has three resonance structures. Choose the stayement that best expains the meaning of those 3 resonance structures. a) Carbonate consists of two single CO bonds and one double CO bond. b) Carbonate consists of one single structure which is an average of the three resonance structures. c) Carbonate quickly flips back and forth between three structures. d) Three different structures for carbonate coexist. #3: When compaing a carbon-carbon double bond (C==C) and a carbon-carbon single bond (C-C), the double bond is a) longer and shorter b) longer and weaker c) shorter and stronger d) shorter and weakerarrow_forward
- Iodine azide, IN3 , adds to alkenes by an electrophilic mechanism similar to that of bromine. If a monosubstituted alkene such as 1-butene is used, only one product results: REFER IMAGE (a) Add lone-pair electrons to the structure shown for IN3 , and draw a second resonance form for the molecule.(b) Calculate formal charges for the atoms in both resonance structures you drew for IN3 in part (a).(c) In light of the result observed when ΙÎ�3 adds to 1-butene, what is the polarity of the I—N3 bond? Propose a mechanism for the reaction using curved arrows to show the electron flow in each steparrow_forwardFor the NO3-1 anion, what are the formal charges on each atom?arrow_forwardHow many resonance structures can be drawn for the hydrogen tellurate ion (HTeO4–) in which the central tellurium atom bears a –1 formal charge and the oxygens bear formal charges of either zero or –1? Enter your answer as a whole number.arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

