
Concept explainers
(a)
Interpretation:
Out of the two distances between two nitrogen atoms, 50 pm or 75 pm, it is to be determined which one represents higher energy.
Concept introduction:
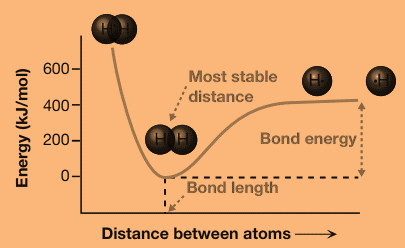
The energy of the molecule depends on the distance between the two atoms. At a certain distance, called the bond length, the energy reaches a minimum. As the distance between the two atoms increases, the energy of the molecule increases and slowly approaches a constant value as the distance increases to infinity. At distances shorter than the bond length, the energy increases very rapidly.
(b)
Interpretation:
Out of the two distances between two nitrogen atoms, 75 pm or 110 pm, it is to be determined which one represents higher energy.
Concept introduction:
The energy of the molecule depends on the distance between the two atoms. At a certain distance, called the bond length, the energy reaches a minimum. As the distance between the two atoms increases, the energy of the molecule increases and slowly approaches a constant value as the distance increases to infinity. At distances shorter than the bond length, the energy increases very rapidly.

(c)
Interpretation:
Out of the two distances between two nitrogen atoms, 110 pm or 150 pm, it is to be determined which one represents higher energy.
Concept introduction:
The energy of the molecule depends on the distance between the two atoms. At a certain distance, called the bond length, the energy reaches a minimum. As the distance between the two atoms increases, the energy of the molecule increases and slowly approaches a constant value as the distance increases to infinity. At distances shorter than the bond length, the energy increases very rapidly.

(d)
Interpretation:
Out of the two distances between two nitrogen atoms, 150 pm or 160 pm, it is to be determined which one represents higher energy.
Concept introduction:
The energy of the molecule depends on the distance between the two atoms. At a certain distance, called the bond length, the energy reaches a minimum. As the distance between the two atoms increases, the energy of the molecule increases and slowly approaches a constant value as the distance increases to infinity. At distances shorter than the bond length, the energy increases very rapidly.

Want to see the full answer?
Check out a sample textbook solution
Chapter 1 Solutions
ORGANIC CHEMISTRY E-BOOK W/SMARTWORK5
- All carbon-to-carbon bond lengths are identical in benzene. Does this argue for or against the presence of C=C bonds in benzene? Explain.arrow_forwardA student draws the picture of ammonia (NH3) in the box below, left, and predicts it will be a flatmolecule with HNH bond angles of exactly 120°. Unfortunately, the student left something out. a. What did the student omit from his drawing? b. What is the actual HNH bond angle of ammonia (based on the draw g above, right)? c. Explain why water, ammonia, and methane (shown below) all have about the same bondangles (close to 109.5°) even though they have different numbers of bonds.arrow_forwardThe cations O2+ and N2+ are formed when molecules of O2 and N2 are subjected to intense, high-energy solar radiation in Earths upper atmosphere. Write the electron configuration for O2+. Predict its bond order and magnetic behavior.arrow_forward
- Calculate the (molar) energy of electrostatic repulsion between two hydrogen nuclei at the separation in H2 (74.1 pm). The result is the energy that must be overcome by the attraction from the electrons that form the bond. Does the gravitational attraction between the nuclei play any significant role? Hint: The gravitational potential energy of two masses is equal to −Gm1m2/r; the gravitational constant G is listed inside the front cover.arrow_forwardThe wavelength at which the O2 molecule most strongly absorbs light is approximately 145 nm. Would a photon whose wavelength is 145 nm haveenough energy to photodissociate O2 whose bond energy is 495 kJ/mol? Would it have enough energy to photoionize O2?arrow_forwardExplain the relationship betweem the Hammond Postulate and the SN1 Reaction ?arrow_forward
- 4..What is the relationship between the stabilization energy of the bonding orbital and Delta E? Proportional? Inversely proportional? Constant?arrow_forwardThe wavelength at which the O2 molecule most stronglyabsorbs light is approximately 145 nm. (a) In which regionof the electromagnetic spectrum does this light fall?(b) Would a photon whose wavelength is 145 nm haveenough energy to photodissociate O2 whose bond energy is495 kJ/mol? Would it have enough energy to photoionize O2?arrow_forwardDetermine the formal weight of CaCl2.arrow_forward
- Qualitatively, how will the potential energy diagram for H2 be altered for N2? Draw one qualitative figure showing the potential energy curve for H2, and then add another curve for N2. Briefly explain how and why they differ.arrow_forwardOn 6b, why are the two Br on the 2nd carbon and not the 3rd??arrow_forwardFor the following molecules: A. XeO2F2 (neutron diffraction experiment by Peterson, Willett and Huston.[1] showed that F-Xe-F bond angle is 180o) B. ClF3 (1) Draw the valid Lewis structure, identify the (2) molecular group geometry, (3) electron group geometry, (4) polarity of the bonds, (5) over-all polarity, (6) number of lone pairs, and (7) number of bonding pairs.arrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning


