
Organic Chemistry
5th Edition
ISBN: 9780078021558
Author: Janice Gorzynski Smith Dr.
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 10, Problem 10.11P
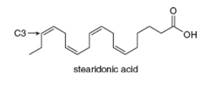
Linolenic acid(Table 10.2) and stearidonic acid are omega-3 fatty acids, unsaturated fatty acids that contain the first double bond located at C3, when numbering begins at the methyl end of the chain. Predict how the melting point of stearidonic acid compares with the melting points of linolenic acid and stearic acids. A current avenue of research is examining the use of soybean oil enriched in stearidonic acid as a healthier alternative to vegetable oils that contain fewer degrees of unsaturation.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
In 1935, J. Bredt, a German chemist, proposed that a bicycloalkene could not have a double bond at a bridgehead carbon unless one of the rings containsat least eight carbons. This is known as Bredt’s rule. Explain why there cannot be a double bond at this position.
Help with the following question. Round your answer to 2 sig figs
(a) Draw a skeletal structure of the anabolic steroid methenolone from the following description. Methenolone contains the tetracyclic steroid skeleton with a carbonyl group at C3, a hydroxyl at C17, a double bond between C1 and C2, and methyl groups bonded to C1, C10, and C13. (b) Add wedges and dashed wedges for all stereogenic centers with thefollowing information: the configuration at C10 is R, the configuration at C13 is S, the configuration at C17 is S, and all substituents at ring fusions are trans to each other. (c) Draw the structure of Primobolan, the product formed when methenolone is treated with CH3(CH2)5COCl and pyridine. Primobolan is an anabolic steroid that can be taken orally or by injection and has been used illegally by well-known Major League Baseball players.
Chapter 10 Solutions
Organic Chemistry
Ch. 10 - Prob. 10.1PCh. 10 - Problem 10.2 How many degrees of unsaturation are...Ch. 10 -
Problem 10.3 How many degrees of unsaturation...Ch. 10 - Give the IUPAC name for each alkene. abcdeCh. 10 - Give the IUPAC name for each polyfunctional...Ch. 10 - Problem 10.6 Label each C-C double bond as E or Z....Ch. 10 - Prob. 10.7PCh. 10 - Prob. 10.8PCh. 10 - Prob. 10.9PCh. 10 - Problem 10.10 Rank the following isomers in order...
Ch. 10 - Linolenic acidTable 10.2 and stearidonic acid are...Ch. 10 - Prob. 10.12PCh. 10 - Problem 10.13 What product is formed when each...Ch. 10 - Prob. 10.14PCh. 10 - Problem 10.15 Draw the products formed when each...Ch. 10 - Prob. 10.16PCh. 10 - Prob. 10.17PCh. 10 - Addition of HBr to which of the following alkenes...Ch. 10 - Problem 10.19 Draw the products, including...Ch. 10 - Prob. 10.20PCh. 10 - Problem 10.21 What two alkenes give rise to each...Ch. 10 - Prob. 10.22PCh. 10 - Problem 10.23 Draw the products of each reaction,...Ch. 10 - Problem 10.24 Draw all stereoisomers formed in...Ch. 10 - Prob. 10.25PCh. 10 - Problem 10.26 What alkylborane is formed from...Ch. 10 - Draw the products formed when each alkene is...Ch. 10 - What alkene can be used to prepare each alcohol as...Ch. 10 - Prob. 10.29PCh. 10 - Draw the products of each reaction using the two...Ch. 10 - Problem 10.31 Devise a synthesis of each compound...Ch. 10 - Give the IUPAC name for each compound. a.b.Ch. 10 - a Label the carbon-carbon double bond in A as E or...Ch. 10 - Prob. 10.34PCh. 10 - 10.35 Calculate the number of degrees of...Ch. 10 - Prob. 10.36PCh. 10 - Label the alkene in each drug as E or Z....Ch. 10 - Give the IUPAC name for each compound. a. c. e. b....Ch. 10 - Prob. 10.39PCh. 10 - 10.40 (a) Draw all possible stereoisomers of, and...Ch. 10 - Prob. 10.41PCh. 10 - 10.42 Now that you have learned how to name...Ch. 10 - Prob. 10.43PCh. 10 - Prob. 10.44PCh. 10 - Prob. 10.45PCh. 10 - Draw the products formed when (CH3)2C=CH2 is...Ch. 10 - What alkene can be used to prepare each alkyl...Ch. 10 - Prob. 10.48PCh. 10 - Draw the constitutional isomer formed in each...Ch. 10 - Prob. 10.50PCh. 10 - Draw all stereoisomers formed in each reaction. a....Ch. 10 - Draw the products of each reaction, including...Ch. 10 - Prob. 10.53PCh. 10 - Prob. 10.54PCh. 10 - Prob. 10.55PCh. 10 - 10.56 Draw a stepwise mechanism for the following...Ch. 10 - Prob. 10.57PCh. 10 - Draw a stepwise mechanism for the conversion of...Ch. 10 - Draw a stepwise mechanism that shows how all three...Ch. 10 - Less stable alkenes can be isomerized to more...Ch. 10 - Prob. 10.61PCh. 10 - Prob. 10.62PCh. 10 - Bromoetherification, the addition of the elements...Ch. 10 - Devise a synthesis of each product from the given...Ch. 10 - 10.65 Draw a synthesis of each compound from...Ch. 10 - 10.66 Explain why A is a stable compound but B is...Ch. 10 - Prob. 10.67PCh. 10 - Prob. 10.68PCh. 10 - 10.69 Lactones, cyclic esters such as compound A,...Ch. 10 - 10.70 Draw a stepwise mechanism for the following...Ch. 10 - 10.71 Like other electrophiles, carbocations add...Ch. 10 - 10.72 Draw a stepwise mechanism for the...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Calculate the lattice energy of CaCl2 using a Born-Haber cycle and data from Appendices F and L and Table 7.5. ...
Chemistry & Chemical Reactivity
Q2. Which statement best defines chemistry?
a. The science that studies solvents, drugs, and insecticides
b. Th...
Introductory Chemistry (6th Edition)
What is the pH range for acidic solutions? For basic solutions?
EBK INTRODUCTION TO CHEMISTRY
Describe the orbitals used in bonding and the bond angles in the following compounds: a. CH3O b. CO2 c. H2CO d....
Organic Chemistry (8th Edition)
22.102 Write the structures of the cis and tram isomers, if any, for the following compounds:
Chemistry: The Molecular Nature of Matter
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Write the IUPAC name of the following molecule: *Lowercase letters only and DO NOT put space in between. Gray balls represent C, white balls represent H, green ball represent F. Do not include stereochemistry.arrow_forward3. Arrange the following four compounds in order of increasing boiling point, and briefly explain your answers: 2-methylhexane, 1-heptanol, heptane, 3,3-dimethylpentane:arrow_forwardIdentify the stereogenic carbon in (S)- and (R)-limonene, rank the substituents around it and rationalize the assignment of their stereochemical configurations. Hint: When ranking carbons that have multiple bonds, consider the bolded carbon of C=C being connected to 2 carbons and the bolded carbon of C≡C being connected to 3 carbons.arrow_forward
- Compound A and compound B are in equilibrium. Write a stepwise mechanism from compound Ato compound B showing ALL intermediates. Use curved arrows to symbolize the flow of electrons to show how each of the intermediates and products are formed. Show all lone pairs and formal charges. Lastly, explain which compound (Aor B) will be in higher concentration.arrow_forwardIn the Diagram below, What is the highest energy conformation of 3-methylpentane when viewed down the 2-3 carbon-carbon bond?arrow_forwardBuild a model of methylcyclohexane, and use the model to complete the following Newmanprojections of methylcyclohexane in the chair conformation: a. When the methyl group is in an axial or equatorial (circle one) position, the molecule is inits lowest potential energy conformation. b. Label one Newman projection above anti and the other gauche to describe the relationshipbetween the methyl group and C3 of the ring. c. In general, which is a lower PE conformation, anti or gauche? d. Explain how your answer to b and c provide an explanation for why it is more favorable fora large group to be in an equatorial than an axial position.arrow_forward
- Write an equation to show the proton transfer between each alkene or cycloalkene and HCl. Where two carbocations are possible, show each.arrow_forwardTrue or False: 1. The eclipsed conformation of a linear alkane IS called cis, while the anti conformation of an alkane IS called trans. 2. In the conversion of open-chain D-glucose to the ring form, the aldehyde carbon (carbon #1) bonds to the oxygen on carbon number 5 to form a ring that is both PYRANOSE and UNLOCKED.arrow_forwardConsider a solvatochromic compound that is blue in water and orange in acetone. Which of the following statements about this compound is true? Group of answer choices The compound exhibits positive solvatochromism The compound exhibits negative solvatochromism In ethanol - a solvent with a polarity between those of water and acetone - the compound would be yellow in color The HOMO is more stabilized by polar solvents than the LUMOarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
ENVIRONMENTAL POLLUTION; Author: 7activestudio;https://www.youtube.com/watch?v=oxtMFmDTv3Q;License: Standard YouTube License, CC-BY