
Concept explainers
Interpretation:
Value of x in
Concept Introduction:
Molecular formula of a compound can be found if the empirical formula and molar mass of the compound is known. The molar mass of compound is divided by the molar mass of the empirical formula in order to obtain the factor which is multiplied with the coefficients of empirical formula in order to obtain the molecular formula.
Lewis structure is used for predicting the shape of molecules. From the steric number obtained in a Lewis structure, the molecular geometry can be predicted. VSEPR model can predict the shape of molecules considering their Lewis structure. Certain rules has to be followed in for the VSEPR model.
- The molecule will have a shape where there is minimal electrostatic repulsion between the valence‑shell electron pairs.
- The forces of repulsion between two lone pairs of electrons will be higher than the repulsion between lone pair and bond pair of electrons. This in turn will be higher than the bond pair‑bond pair of electrons.
The hybridized orbitals and the steric number can be related as shown below;
| Steric number | Hybridized orbital |
| 2 | |
| 3 | |
| 4 | |
| 5 | |
| 6 |
Explanation of Solution
Molecular formula:
The percentage composition of the compound was
Moles of carbon:
Moles of hydrogen:
Moles of oxygen:
Empirical formula can be obtained by dividing the moles of each element with the least mole. This is done as follows;
Therefore, the ratio of the element can be given as
Molar mass of compound is given as
Molar mass of the empirical formula is calculated as follows;
Molar mass of the compound is divided by the molar mass of empirical formula in order to obtain the factor as shown below;
The coefficient of empirical formula is multiplied by the factor
Therefore, the molecular formula of compound is
Lewis structure of first compound:
The compound is analyzed as
The total number of valence electrons in
Skeletal structure of
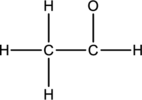
A total of twelve electrons are involved in the skeletal structure. Six electrons are placed over the oxygen atom as lone pair of electrons. Thus the Lewis structure for
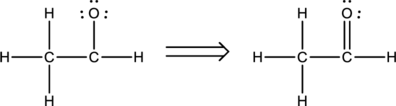
Bond angles and hybridization:
The first carbon atom does not have any lone pair of electrons while it is bonded to four other atoms. Therefore, the steric number is calculated as shown below;
As the steric number is four, the arrangement is tetrahedral and the bond angle will be
The second carbon atom does not have any lone pair of electrons while it is bonded to three other atoms. Therefore, the steric number is calculated as shown below;
As the steric number is three, the arrangement is trigonal planar and the bond angle will be
Lewis structure of second compound:
The compound is analyzed as
The total number of valence electrons in
Skeletal structure of
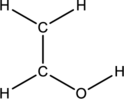
A total of twelve electrons are involved in the skeletal structure. Four electrons are placed over the oxygen atom as lone pair of electrons. Thus the Lewis structure for
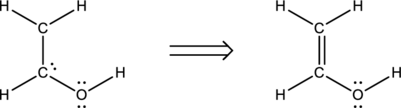
Bond angles and hybridization:
The first carbon atom does not have any lone pair of electrons while it is bonded to three other atoms. Therefore, the steric number is calculated as shown below;
As the steric number is three, the arrangement is trigonal planar and the bond angle will be
The second carbon atom does not have any lone pair of electrons while it is bonded to three other atoms. Therefore, the steric number is calculated as shown below;
As the steric number is three, the arrangement is trigonal planar and the bond angle will be
The oxygen atom does have two lone pair of electrons while it is bonded to two other atoms. Therefore, the steric number is calculated as shown below;
As the steric number is four, the arrangement is tetrahedral and the bond angle will be
Want to see more full solutions like this?
Chapter 10 Solutions
Chemistry: Principles and Practice
- Gamma hydroxybutyric acid, GHB, infamous as a date rape drug, is used illicitly because of its effects on the nervous system. The condensed molecular formula for GHB is HO(CH2)3COOH. (a) Write the Lewis structure for GHB. (b) Identify the hybridization of the carbon atom in the CH2 groups and of the terminal carbon. (c) Is hydrogen bonding possible in GHB? If so, write Lewis structures to illustrate the hydrogen bonding. (d) Which carbon atoms are involved in sigma bonds? In pi bonds? (e) Which oxygen atom is involved in sigma bonds? In pi bonds?arrow_forwardThe hybridization of the two carbon atoms differs in an acetic acid, CH3COOH, molecule. (a) Designate the correct hybridization for each carbon atom in this molecule. (b) What is the approximate bond angle around each carbon?arrow_forwardIn Chapter 6, we study a group of organic cations called carbocations. Following is the structure of one such carbocation, the tert-butyl cation. (a) How many electrons are in the valence shell of the carbon bearing the positive charge? (b) Using VSEPR, predict the bond angles about this carbon. (c) Given the bond angle you predicted in (b), what hybridization do you predict for this carbon?arrow_forward
- For each of the following molecules, state the bond angle (or bond angles, as appropriate) that you would expect to see on the central atom based on the simple VSEPR model. Would you expect the actual bond angles to be greater or less than this? a CCl4 b SCl2 c COCl2 d AsH3arrow_forwardIdentify the type of hybridization, approximate bond angles for the N, C, and O atoms, and shortest carbon-to-oxygen bond length in alanine, an amino acid, whose Lewis structure isarrow_forwardAspirin, or acetylsalicylic acid, has the formula C9H8O4 and the skeleton structure (a) Complete the Lewis structure and give the number of bonds and bonds in aspirin. (b) What is the hybridization about the CO2H carbon atom (colored blue)? (c) What is the hybridization about the carbon atom in the benzene-like ring that is bonded to an oxygen atom (colored red)? Also, what is the hybridization of the oxygen atom bonded to this carbon atom?arrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning





