
Concept explainers
(a)
Interpretation:
The probability for the particle having wavefunction
Concept introduction:
For the normalization of the wavefunction, the wavefunction is integrated as a product of its conjugate over the entire limits. It is expressed by the equation as given below.
Where,
•
•
•
Answer to Problem 10.27E
The probability for the particle having wavefunction
Explanation of Solution
For the probability of the wavefunction the expression is as follows.
Where,
•
•
•
•
Substitute the values in the above equation as follows.
The above equation is simplified as given below.
The probability for the particle having wavefunction
(b)
Interpretation:
The probability for the particle having wavefunction
Concept introduction:
For the normalization of the wavefunction, the wavefunction is integrated as a product of its conjugate over the entire limits. It is expressed by the equation as given below.
Where,
•
•
•
Answer to Problem 10.27E
The probability for the particle having wavefunction
Explanation of Solution
For the probability of the wavefunction the expression is as follows.
Where,
•
•
•
•
Substitute the values in the above equation as follows.
The above equation is simplified as follows.
The probability for the particle having wavefunction
(c)
Interpretation:
The probability for the particle having wavefunction
Concept introduction:
For the normalization of the wavefunction, the wavefunction is integrated as a product of its conjugate over the entire limits. It is expressed by the equation as given below.
Where,
•
•
•
Answer to Problem 10.27E
The probability for the particle having wavefunction
Explanation of Solution
For the probability of the wavefunction the expression is as follows.
Where,
•
•
•
•
Substitute the values in the above equation as follows.
The above equation is simplified as follows.
The probability for the particle having wavefunction
(d)
Interpretation:
The probability for the particle having wavefunction
Concept introduction:
For the normalization of the wavefunction, the wavefunction is integrated as a product of its conjugate over the entire limits. It is expressed by the equation as given below.
Where,
•
•
•
Answer to Problem 10.27E
The probability for the particle having wavefunction
Explanation of Solution
For the probability of the wavefunction the expression is as follows.
Where,
•
•
•
•
Substitute the values in the above equation as follows.
The above equation is simplified as given below.
The probability for the particle having wavefunction
(e)
Interpretation:
The probability for the particle having wavefunction
Concept introduction:
For the normalization of the wavefunction, the wavefunction is integrated as a product of its conjugate over the entire limits. It is expressed by the equation as given below.
Where,
•
•
•
Answer to Problem 10.27E
The probability for the particle having wavefunction
Explanation of Solution
For the probability of the wavefunction the expression is as follows.
Where,
•
•
•
•
Substitute the values in the above equation as follows.
The above equation is simplified as follows.
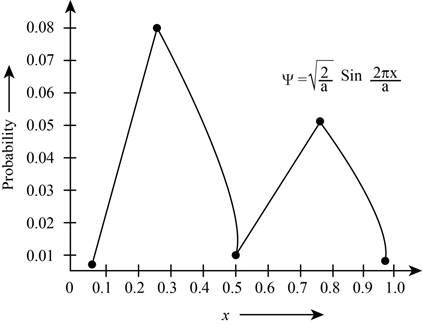
The plot the probabilities versus

Figure 1
The plot shows the probability for the given wave function. According to this plot, the probability of finding the particle is maximum in the range of
The probability for the particle having wavefunction
Want to see more full solutions like this?
Chapter 10 Solutions
Physical Chemistry
- What is the physical explanation of the difference between a particle having the 3-D rotational wavefunction 3,2 and an identical particle having the wavefunction 3,2?arrow_forwardWhat values of J may occur in the terms (i) 1S, (ii) 2P, (iii) 3P? How many states (distinguished by the quantum number MJ) belong to each level?arrow_forwardTo what speed must a proton be accelerated from rest for it to have a de Broglie wavelength of 100 pm? What accelerating potential difference is needed?arrow_forward
- Functions of the form sin(nπx/L), where n = 1, 2, 3 …, are wavefunctions in a region of length L (between x = 0 and x = L). Show that the wavefunctions with n = 1 and 2 are orthogonal; you will find the necessary integrals in the Resource section.arrow_forwardFind an expression for the value of n of a particle of mass m in a one-dimensional box of length L such that the energy of the level is equal to the mean energy of thermal motion (1/2kT). Calculate the value of n for the case of an argon atom in a box of length 0.1 cm at 298 K.arrow_forwardA certain metal must absorb radiation with a wavelength no larger than 275 nm in order to eject an electron from its surface via the photoelectric effect. Calculate the work function of this metal in units of kJ mol-1.arrow_forward
- Calculate the size of the quantum involved in the excitation of an electronic motion of frequency 1.0 x 1015 Hz. Ans: 400 kJ/molarrow_forwardNormalize the wave function ψ= A sin (nπ/a x) by finding the value of the constant A when the particle is restricted to move in one dimensional box of width ‘a’.arrow_forward8C.5 (a) use the data in 8C.4 (a) to calculate the energy needed excite a CH4 molecule from a state with l=1 to a state with l=2arrow_forward
- Calculate the energy of the quantum involved in the excitation of (i) an electronic oscillation of period 1.0 fs, (ii) a molecular vibration of period 10 fs, (iii) a pendulum of period 1.0 s. Express the results in joules and kilojoules per mole.arrow_forwardTo what speed must an electron be accelerated from rest for it to have a de Broglie wavelength of 100 pm? What accelerating potential difference is needed?arrow_forwardCalculate the expectation values of x and x2 for a particle in the state with n = 1 in a one-dimensional square-well potential.arrow_forward
 Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
