
Concept explainers
(a)
Interpretation:
The most polar bond of methanol should be identified and polarity described with symbols
Concept Introduction:
Polarity of any molecule depends on electronegativity of atoms that forms covalent bonds. Depending upon the difference in electronegativity of atoms, polar or nonpolar molecule is determined. Polar molecule has electronegativity between 0.5 and 1.4 whereas in nonpolar molecule, it is less than 0.5.
Answer to Problem 10.49P
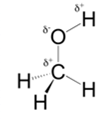
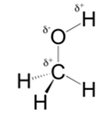
In methanol, most polar bond is O-H bond with electronegativity difference of 1.4 and oxygen atom becomes partial negative charge atom whereas hydrogen becomes partial positive charge atom.
Explanation of Solution
Polar covalent bonds contains more atoms with high electronegativity and acquire more partial negative charge represented as delta minus
In methanol, most polar bond is O-H with electronegativity difference of 1.4 and oxygen atom becomes partial negative charge atom whereas hydrogen becomes partial positive charge atom.
C-O bond have 1.0 electronegativity but it is less polar when compare to O-H bond.
(b)
Interpretation:
The most polar bond of methylamine should be identified and polarity described with symbols
Concept Introduction:
Polarity of any molecule depends on electronegativity of atoms that forms covalent bonds. Depending upon the difference in electronegativity of atoms, polar or nonpolar molecule is determined. Polar molecule has electronegativity between 0.5 and 1.4 whereas in nonpolar molecule, it is less than 0.5.
Answer to Problem 10.49P
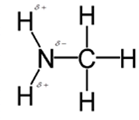
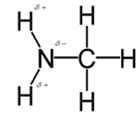
In methylamine, most polar bond is N-H bond with electronegativity difference of 0.9 and nitrogen atom becomes partial negative charge atom whereas hydrogen becomes partial positive charge atom.
Explanation of Solution
Polar covalent bonds contains more atoms with high electronegativity and acquire more partial negative charge represented as delta minus
In methylamine, most polar bond is N-H with electronegativity difference of 0.9 and nitrogen atom becomes partial negative charge atom whereas hydrogen becomes partial positive charge atom.
C-H bond is nonpolar because of electronegativity of 0.4 only.
(c)
Interpretation:
The most polar bond of 2-aminoethathiol should be identified and polarity described with symbols
Concept Introduction:
Polarity of any molecule depends on electronegativity of atoms that forms covalent bonds. Depending upon the difference in electronegativity of atoms, polar or nonpolar molecule is determined. Polar molecule has electronegativity between 0.5 and 1.4 whereas in nonpolar molecule, it is less than 0.5.
Answer to Problem 10.49P
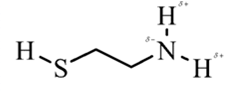
In 2-aminoethathiol, most polar bond is N-Hbond with electronegativity difference of 0.9 and nitrogen atom becomes partial negative charge atom whereas hydrogen becomes partial positive charge atom.
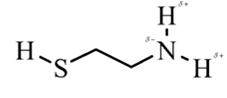
Explanation of Solution
Polar covalent bonds contains more atoms with high electronegativity and acquire more partial negative charge represented as delta minus
In 2-aminoethanethiol, most polar bond is N-H with electronegativity difference of 0.9 and nitrogen atom becomes partial negative charge atom whereas hydrogen becomes partial positive charge atom.
The bonds S-H, C-C and C-H have electronegativity difference are 0.4, 0, and 0.4 respectively.
(d)
Interpretation:
The most polar bond of acetone should be identified and polarity described with symbols
Concept Introduction:
Polarity of any molecule depends on electronegativity of atoms that forms covalent bonds. Depending upon the difference in electronegativity of atoms, polar or nonpolar molecule is determined. Polar molecule has electronegativity between 0.5 and 1.4 whereas in nonpolar molecule, it is less than 0.5.
Answer to Problem 10.49P
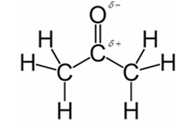
In acetone, most polar bond is C=O with electronegativity difference of 1.0 and oxygen atom becomes partial negative charge atom whereas carbon becomes partial positive charge atom.
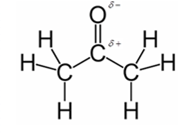
Explanation of Solution
Polar covalent bonds contains more atoms with high electronegativity and acquire more partial negative charge represented as delta minus
In acetone, most polar bond is C=O with electronegativity difference of 1.0 and oxygen atom becomes partial negative charge atom whereas carbon becomes partial positive charge atom.
Other bonds present in acetone are C-H and C-C with electronegativity of 0.4 and 0 respectively.
(e)
Interpretation:
The most polar bond of formaldehyde should be identified and polarity described with symbols
Concept Introduction:
Polarity of any molecule depends on electronegativity of atoms that forms covalent bonds. Depending upon the difference in electronegativity of atoms, polar or nonpolar molecule is determined. Polar molecule has electronegativity between 0.5 and 1.4 whereas in nonpolar molecule, it is less than 0.5.
Answer to Problem 10.49P
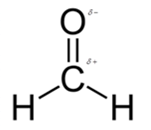
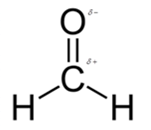
In formaldehyde, most polar bond is C=O with electronegativity difference of 1.0 and oxygen atom becomes partial negative charge atom whereas carbon becomes partial positive charge.
Explanation of Solution
Polar covalent bonds contains more atoms with high electronegativity and acquire more partial negative charge represented as delta minus
In formaldehyde, most polar bond is C=O with electronegativity difference of 1.0 and oxygen atom becomes partial negative charge atom whereas carbon becomes partial positive charge atom.
The bond C-H has electronegativity difference of 0.4 and it is a nonpolar bond.
(f)
Interpretation:
The most polar bond of acetic acid should be identified and polarity described with symbols
Concept Introduction:
Polarity of any molecule depends on electronegativity of atoms that forms covalent bonds. Depending upon the difference in electronegativity of atoms, polar or nonpolar molecule is determined. Polar molecule has electronegativity between 0.5 and 1.4 whereas in nonpolar molecule, it is less than 0.5.
Answer to Problem 10.49P
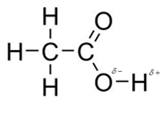
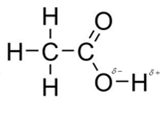
In acetic acid, most polar bond is O-H with electronegativity difference of 1.4 and oxygen atom becomes partial negative charge atom whereas hydrogen becomes partial positive charge atom.
Explanation of Solution
Polar covalent bonds contains more atoms with high electronegativity and acquire more partial negative charge represented as delta minus
In acetic acid, most polar bond is O-H with electronegativity difference of 1.4 and oxygen atom becomes partial negative charge atom whereas hydrogen becomes partial positive charge atom.
The other bonds are C-C, C=O and C-H with electronegativity of 0, 1.0 and 0.4 but bond O-H has more electronegativity as compared to other bonds.
Want to see more full solutions like this?
Chapter 10 Solutions
Introduction to General, Organic and Biochemistry
- 3-122 Some of the following structural formulas are incorrect because they contain one or more atoms that do not have their normal number of covalent bonds. Which structural formulas are incorrect, and which atom or atoms in each have the incorrect number of bonds?arrow_forward3-31 Why does electronegativity generally increase going from left to right across a row of the Periodic Table?arrow_forward3-106 Consider the structure of Penicillin G shown below, an antibiotic used to treat bacterial infections caused by gram-positive organisms, derived from Penicillium fungi: (a) Identify the various types of geometries present in each central atom using VSEPR theory. (b) Determine the various relative bond angles associated with each central atom using VSEPR theory (c) Which is the most poiar bond in Penicillin G? (d) Would you predict Penicillin G to be polar or nonpolar?arrow_forward
- 3-25 Why are carbon and silicon reluctant to form ionic bonds?arrow_forward3-67 Why does nitrogen have three bonds and one unshared pair of electrons in covalent compounds?arrow_forward3-107 Ephedrine, a molecule at one time found in the dietary supplement ephedra, has been linked to adverse health reactions, such as heart attacks, strokes, and heart palpitations. The use of ephedra in dietary supplements is now banned by the FDA. (a) Which is the most polar bond in ephedra? (b) Would you predict ephedra to be polar or nonpolar?arrow_forward
- 3-120 Vinyl chloride is the starting material for the production of poly(vinyl chloride), abbreviated PVC. Its recycling code is “V”. The major use of PVC is for tubing in residential and commercial construction (Section 12-7). (a) Complete the Lewis structure for vinyl chloride by showing all unshared pairs of electrons. (b) Predict the HCH, HCC, and ClCH bond angles in this molecule. (c) Does vinyl chloride have polar bonds? Is it a polar molecule? Does it have a dipole?arrow_forward3-63 What is the difference between (a) a bromine atom, (b) a bromine molecule, and (c) a bromide ion? Draw the Lewis structure for each.arrow_forwardIs violet light (with a wavelength of 400 nm) sufficiently energetic to break a carbon-carbon single bond (the average C-C bond energy is 346 kJ/mol)?arrow_forward
- 3-127 Amoxicillin is an antibiotic used to treat bacterial infections caused by susceptible microorganisms. Consider the skeletal structure of amoxicillin (y refer to the structure at bottom of page). Where all the bonded atoms are shown but double bonds, triple bonds, and/or lone pairs are missing: (a) Complete the structure of amoxicillin. (b) Identify the various types of geometries present in each central atom using VSEPR theory (e) Determine the various relative bond angles as sociated with each central atom using VSEPR theory. (d) What is the most polar bond in Amoxicillin? (e) Would you predict amoxicillin to be polar or nonpolar? (f) Is amoxicillin expected to possess resonance? Explain why or why not. V Chemical structure for problem 3-127arrow_forward3-64 Acetylene (C2H2), hydrogen cyanide (HCN), and nitrogen (N2) each contain a triple bond. Draw a Lewis structure for each molecule. Which of these are polar molecules, and which are nonpolar molecules?arrow_forwardA becuas eit has 3 bonds?arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning


