
Concept explainers
(a)
Interpretation:
The respective reactant (methyl substituted piperidine) and respective product (diene) should be given.
Concept introduction:
Generally
These quaternary ammonium salt under goes an elimination reaction easily.
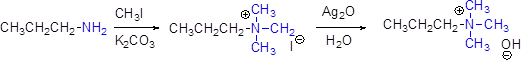
Hofmann elimination:
Quaternary ammonium ion undergoes elimination when using strong base like hydroxide ion this reaction is called as Hofmann elimination.
In the Hofmann elimination, abstraction of proton form β- carbon atom which is having more number of hydrogen to result the elimination product.

(b)
Interpretation:
The respective reactant (methyl substituted piperidine) and respective product (diene) should be given.
Concept introduction:
Hofmann elimination:
Quaternary ammonium ion undergoes elimination when using strong base like hydroxide ion this reaction is called as Hofmann elimination.
In the Hofmann elimination, abstraction of proton form β- carbon atom which is having more number of hydrogen to result the elimination product.

(c)
Interpretation:
The respective reactant (methyl substituted piperidine) and respective product (diene) should be given.
Concept introduction:
Hofmann elimination:
Quaternary ammonium ion undergoes elimination when using strong base like hydroxide ion this reaction is called as Hofmann elimination. Proton abstraction is takes place in β- carbon atom which is having more number of hydrogen.
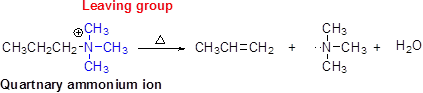
(d)
Interpretation:
The respective reactant (methyl substituted piperidine) and respective product (diene) should be given.
Concept introduction:
Generally amines can’t undergo elimination reaction, therefore amines can be converted in to quaternary ammonium halide by treating with ethyl iodide in basic solution of potassium carbonate, and this ammonium halide is treating with silver oxide to give ammonium hydroxide salt.
These quaternary ammonium salt under goes an elimination reaction easily.
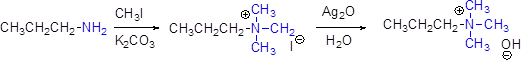
Hofmann elimination:
Quaternary ammonium ion undergoes elimination when using strong base like hydroxide ion this reaction is called as Hofmann elimination.
In the Hofmann elimination, abstraction of proton form β- carbon atom which is having more number of hydrogen to result the elimination product.

Want to see the full answer?
Check out a sample textbook solution
Chapter 10 Solutions
ORGANIC CHEMISTRY (LL)-W/MOD.MASTERING.
- Write the monobromination products of 1,4-dimethylcyclohexane and calculate the percentages of each product. Which is the major product?arrow_forwardAlkyl halides undergo nucleophilic substitution and elimination reactions. When the kinetics of the reaction are measured, if the rate of the reaction is found to be dependent only upon the concentration of the alkyl halide the reaction is first order. The substitution reaction is thus termed SN1, and the elimination reaction is termed E1. These reactions are unimolecular and occur in two steps. The first step is rate-limiting and involves the loss of the leaving group to form a carbocation. In the second, fast, step the nucleophile adds to the carbocation in the SN1 reaction or elimination occurs to give an alkene in the E1 reaction. Because the carbocation is planar, the nucleophile can add to either face and therefore racemization is usually observed although solvent effects can influence this somewhat. E1 elimination follows Zaitsev’s rule and typically yields the most substituted alkene as the major product. Conditions which favor the SN1/E1 pathway include the use of a weak…arrow_forwardAlkyl halides undergo nucleophilic substitution and elimination reactions. When the kinetics of the reaction are measured, if the rate of the reaction is found to be dependent only upon the concentration of the alkyl halide the reaction is first order. The substitution reaction is thus termed SN1, and the elimination reaction is termed E1. These reactions are unimolecular and occur in two steps. The first step is rate-limiting and involves the loss of the leaving group to form a carbocation. In the second, fast, step the nucleophile adds to the carbocation in the SN1 reaction or elimination occurs to give an alkene in the E1 reaction. Because the carbocation is planar, the nucleophile can add to either face and therefore racemization is usually observed although solvent effects can influence this somewhat. E1 elimination follows Zaitsev’s rule and typically yields the most substituted alkene as the major product. Conditions which favor the SN1/E1 pathway include the use of a weak…arrow_forward
- The rate law for addition of Br2 to an alkene is first orderin Br2 and first order in the alkene. Does this informationsuggest that the mechanism of addition of Br2 to analkene proceeds in the same manner as for addition of HBr?Explain.arrow_forwardThe compound whose structure is shown here is acetyl acetone. It exists in two forms:the enol form and the keto form The molecule reacts with OH–to form an anion, [CH3COCHCOCH3] (often abbreviatedacac–for acetylacetonate ion). One or the most interesting aspects of this anion is thatone or more of them can react with transition metal cations to give stable, highlycolored compounds (a) Are the keto and enol forms of acetylacetone resonance forms? Explain youranswer.(b) What is the hybridization or each atom (except H) in the enol form? What changesin hybridization occur when it is transformed into the keto form?(c) What are the electron-pair geometry and molecular geometry around each C atomin the keto and enol forms? What changes in geometry occur when the keto formchanges to the enol form?(d) Draw three possible resonance structures for the acac–ion.(e) Is cis-trans isomerism possible in either the enol or the keto form of acetylacetone?(f) Is the enol form of acetylacetone polar?…arrow_forwardDiels–Alder reaction of a monosubstituted diene (such as CH2=CH– CH=CHOCH3) with a monosubstituted dienophile (such as CH2=CHCHO) gives a mixture of products, but the 1,2-disubstituted product often predominates. Draw the resonance hybrid for each reactant, and use the charge distribution of the hybrids to explain why the 1,2-disubstituted product is the major product.arrow_forward
- Uemura and coworkers studied a time dependent Diels-Alder reaction which first formed the endo product as the major organic product and with time produced the exo product (J. Org. Chem. 2018, 83, 9300−9304). Show the endo and exo product for the reaction below. Which is the thermodynamic product and which is the kinetic product? Explain your reasoning.arrow_forwardWhich of the statement is INCORRECT? a. The increase in stability of 2,4-hexadiene over 1,3-hexadiene is due to the increased double bond substitution of the former. b. The stabilization of dienes by conjugation is less pronounced than the aromatic stabilization of benzene. c. Resonance description in alkenes usually involves charge separation. d. Higher energy pi-orbitals often have decreasing number of nodes.arrow_forwardThe heat of hydrogenation of cis-2,2,5,5-tetramethyl-3-hexene is -154 kJ (-36.7 kcal)/ mol, while that of the trans isomer is only -113 kJ (-26.9 kcal)/mol. Q.) If a catalyst could be found that allowed equilibration of the cis and trans isomers at room temperature (such catalysts do exist), what would be the ratio of trans to cis isomers?arrow_forward
- Diels—Alder reaction of a monosubstituted diene (such as CH2 = CH – CH = CHOCH3) with a monosubstituted dienophile (such as CH2 = CHCHO) gives a mixture of products, but the 1,2-disubstituted product often predominates. Draw the resonance hybrid for each reactant and use the charge distribution of the hybrids to explain why the 1,2-disubstituted product is the major product.arrow_forwarda. How many monochlorination products can be obtained from the radical chlorination of methylcyclohexane? Disregard stereoisomers.b. Which product would be obtained in greatest yield? Explain.c. How many monochlorination products would be obtained if all stereoisomers are included?arrow_forwardAcrolein and 1,3-cyclohexadiene react in a one-step concerted manner to yield a single product. Give the structure of the product. What kind of reaction is this an example of? In terms of this reaction, how would you classify acrolein? How would you classify 1,3-cyclohexadiene? Hint: acrolein is not a systematic name so you may need to look up its structure if you are not already familiar with it.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

