
(a)
Interpretation:
The primary amine starting material should be identified to form a 4-Methyl-2-pentene via Hofmann degradation.
Concept introduction:
Generally
In the reaction, quaternary ammonium halide can be converted in to quaternary ammonium hydroxide by using aqueous silver oxide.
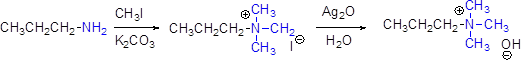
Hofmann elimination:
Quaternary ammonium ion undergoes elimination when using strong base like hydroxide ion this reaction is called as Hofmann elimination.
In the reaction, proton abstraction is takes place in β- carbon atom, which is having more number of hydrogen.

(b)
Interpretation:
The primary amine starting material should be identified to form a 3-Methyl-l-butene via Hofmann degradation.
Concept introduction:
Hofmann elimination:
Quaternary ammonium ion undergoes elimination when using strong base like hydroxide ion this reaction is called as Hofmann elimination.
In the reaction, proton abstraction is takes place in β- carbon atom, which is having more number of hydrogen.

(c)
Interpretation:
The secondary amine starting material should be identified to form a 2-Methyl-1·3-butadiene via Hofmann degradation.
Concept introduction:
Hofmann elimination:
Quaternary ammonium ion undergoes elimination when using strong base like hydroxide ion this reaction is called as Hofmann elimination.
In the reaction, proton abstraction is takes place in β- carbon atom, which is having more number of hydrogen.

Want to see the full answer?
Check out a sample textbook solution
Chapter 10 Solutions
ORGANIC CHEMISTRY (LL)-W/MOD.MASTERING.
- Several additional amine syntheses are effectively limited to making primary amines. The reduction of azides and nitrocompounds and the Gabriel synthesis leave the carbon chain unchanged. Formation and reduction of a nitrile adds onecarbon atom. Show how these amine syntheses can be used for the following conversions. (c) 1@bromo@3@phenylheptane S 3@phenylheptan@1@amine (d) 1@bromo@3@phenylheptane S 4@phenyloctan@1@aminearrow_forwardGive an inference and observation when the compound hexane is subjected into Tollen's test and Iodoform Testarrow_forwardPrimary amines can also be prepared by the reaction of an alkyl halide with azide ion, followed by catalytic hydrogenation. What advantage do this method and the Gabriel synthesis have over the synthesis of a primaryamine using an alkyl halide and ammonia?arrow_forward
- The Stork reaction is a condensation reaction between an enamine donor and an α,β-unsaturated carbonyl acceptor. The overall reaction consists of a three-step sequence of formation of an enamine from a ketone, Michael addition to an α,β-unsaturated carbonyl compound, and hydrolysis of the enamine in dilute acid to regenerate the ketone. Consider the Stork reaction between acetophenone and 3-buten-2-one. Draw the structure of the product of the enamine formed between acetophenone and pyrrolidine. Draw the structure of the Michael addition product. Draw the structure of the final product.arrow_forwardAcetic anhydride is hydrolyzed by water to provide two molecules of acetic acid. Propose amechanism for this reaction.arrow_forwardAcyl Compounds: Acetyl chloride, Acetic anhydride, Ethyl benzoate and Benzamide1. Which among the acyl compounds above are easily hydrolyzed (do not require heating)? 2. Which of them are not easily hydrolyzed (require heating)? 3. Based on the results, arrange the following compound types in order of decreasing ease of hydrolysis: acid halides, acid anhydrides, esters, and amides. Use > in your arrangementarrow_forward
- Amino acids such as glycine are the building blocks of large molecules called proteins that give structure to muscle, tendon, hair, and nails. a.) Explain why glycine does not actually exist in the form with all atomsuncharged, but actually exists as a salt called a zwitterion. b.) What product is formed when glycine is treated with concentratedHCl?c.) What product is formed when glycine is treated with NaOH?arrow_forwardpresent the Fisher esterification mechanism of the reaction of acetic acid with pentanol in the presence of an acid catalyst, sulfuric acid to form pentyl acetate and explain the mechanismarrow_forwarda) Put these three common types of carbonyl compound in order of decreasing reactivity ester amide acid chloride b) For the least reactive, show the interconversion to its other resonance form: How does this electron delocalisation make it stable? c) For the most reactive, draw the mechanism of its undergoing hydrolysis (reaction with H2O): Why makes this type of carbonyl so reactive to nucleophiles?arrow_forward
- In biochemical reactions, decarboxylation of carboxylic acids typically takes place for-keto carboxylic acids. Justify a rational why nature opted for-keto carboxylic acid decarboxylation. Among the following types of biochemical reactions, ester hydrolysis, rearrangement reactions, water elimination reactions, and anhydride hydrolyses, which one is the most favorable one. Rank the above reactions types in the order of being the most to least favorable reactionarrow_forwardSeveral sulfonylureas, a class of compounds containing RSO2NHCONHR, are useful drugs as orally active replacements for injected insulin in patients with adult-onset diabetes. These drugs decrease blood glucose concentrations by stimulating b cells of the pancreas to release insulin and by increasing the sensitivity of insulin receptors in peripheral tissues to insulin stimulation. Tolbutamide is synthesized by the reaction of the sodium salt of p-toluenesulfonamide and ethyl N-butylcarbamate . Propose a mechanism for this step.arrow_forwardWhat reaction sequence will form dibenylamine from benzoic acid in high yieldarrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


