
(a)
Interpretation:
The constitutional isomer, which is formed greatest yield in the given reaction should be given.
Concept introduction:
Dehydration reaction:
Removal of water molecule from the reaction when the alcohol is treated with strong acid like sulfuric acid is known as dehydration reaction, for example
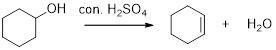
Alcohols are react with acids like hydrochloric acid or hydrobromic to yields the corresponding carbocation intermediates, this carbocation intermediate undergoes elimination reaction to give a corresponding
The stability of carbocation is given below,
Tertiary carbocation is more stable than the secondary and primary.
Cis–trans isomerism (or) geometric isomerism or configurational isomerism:
The
The functional groups are in opposite to each other in the carbon chain is called trans isomer.
Two similar functional groups are in same side which is called as Z-isomer.
Two similar functional groups are opposite side which is called as E-isomer.
Stereoisomers: same molecular formula and sequence of bonded atoms (constitution), but differ in the three-dimensional orientations of their atoms in space.
(b)
Interpretation:
The stereoisomer, which is formed greatest yield in the given reaction should be given.
Concept introduction:
Dehydration reaction:
Dehydration reaction:
Removal of water molecule from the reaction when the alcohol is treated with strong acid like sulfuric acid is known as dehydration reaction, for example
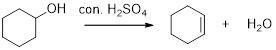
Alcohols are react with acids like hydrochloric acid or hydrobromic to yields the corresponding carbocation intermediates, this carbocation intermediate undergoes elimination reaction to give a corresponding alkene as a product.
The stability of carbocation is given below,
Tertiary carbocation is more stable than the secondary and primary.
Cis–trans isomerism (or) geometric isomerism or configurational isomerism:
The functional groups are in the same side of the carbon chain is called cis isomer.
The functional groups are in opposite to each other in the carbon chain is called trans- isomer.
Two similar functional groups are in same side which is called as Z-isomer.
Two similar functional groups are opposite side which is called as E-isomer.
Stereoisomers: same molecular formula and sequence of bonded atoms (constitution), but differ in the three-dimensional orientations of their atoms in space.
Want to see the full answer?
Check out a sample textbook solution
Chapter 10 Solutions
ORGANIC CHEMISTRY (LL)-W/MOD.MASTERING.
- Name the type of reaction and provide a possible reagent assign all the stereocentres found in the compounds via cahn ingold prelog rules define the relation between compound. D E F G identify the 2 stereoisomers likely to be formed in compound carrow_forwardWhat are the intermediates J, K and L? What regiochemistry and stereochemistry is involved in each step? Why is M the only stereoisomer formed?arrow_forwardShow how to make these deuterium-labeled compounds, using CD3MgBr and D2O as your sources of deuterium, and anynon-deuterated starting materials you wish.(a) CH3CH(OD)CD3arrow_forward
- Treating 4-penten-1-ol with bromine in water forms a cyclic bromoether. Account for the formation of this product rather than a bromohydrin as was formed in Problem 6.33arrow_forwarda.Draw three-dimensional representations for all stereoisomers of 2chloro-3-methylpentane, and label pairs of enantiomers. b. Considering dehydrohalogenation across only C2 and C3, draw the E2 product that results from each of these alkyl halides. How many different products have you drawn? c. How are these products related to each other?arrow_forwardWhich alkyl halide from the following pair is (i) Chiral and (ii) undergoes SN1 reaction faster?(a) (CH3)2CBr(b) CH3CH2CHBrCH3arrow_forward
- What allylic alcohol and DET isomer are needed to make each chiralepoxide using a Sharpless asymmetric epoxidation reaction?arrow_forwardCompound W shows stereoisomerism. Why are such isomers formed in approximately equimolar quantities no matter the synthetic pathway used for the preparation of the compound?arrow_forwardWhen the optically active compound shown below reacts with HCl, two major products are formed: one optically active, and the other optically inactive. Draw each product. Which product is major?arrow_forward
- Draw the SNl or SN2 reaction with CN for the following molecules t butylchloride , n - butylbromide , 2 -bromobutane . Explain in which mechanism it will react and why ?arrow_forward1. Why P(CH₂CH₃)₃ dissociiation is faster than PF3?2. What is the product from cis-[PtCl2(NH3)2] + 2py and explainarrow_forwardwrite the correct product for each and indicate stereochemistry if necessary.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


