
Concept explainers
(a)
Interpretation:
The detailed mechanism for the production of given compounds from the respective alkyne is to be drawn.
Concept introduction:
The
Answer to Problem 11.39P
The detailed mechanism for the given reaction with a major product is:
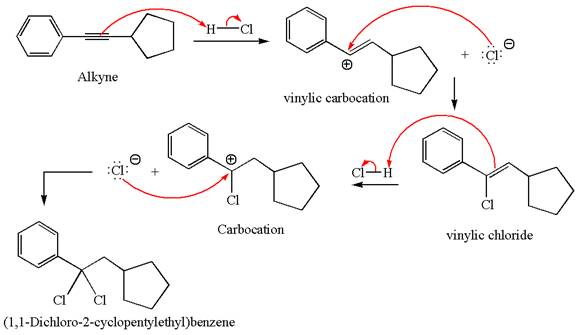
Explanation of Solution
The structure for the given compound
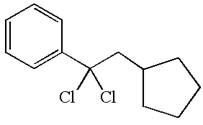
The given compound is germinal dichloride, and thus can be produced by electrophilic addition of an excess of
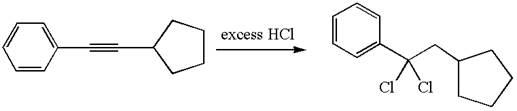
In the given reaction the alkyne is the electron rich site and the hydrogen from the
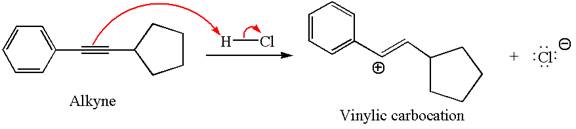
In the second step, the chloride ion acts as a nucleophile and attacks at vinylic carbocation forming vinylic chloride product.
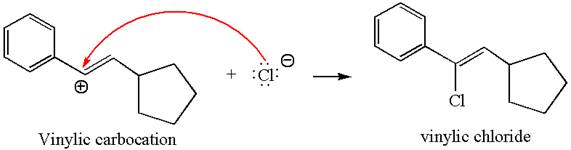
The vinylic chloride again undergoes the addition of
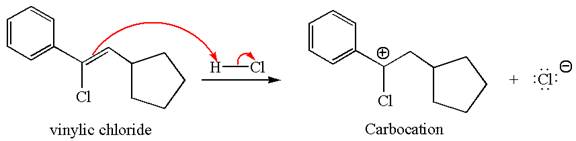
This carbocation further reacts with nucleophilic chloride ion to form a germinal dichloride product.
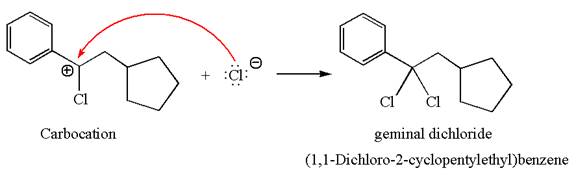
The detailed mechanism is drawn for the given reaction with showing the formation of stable carbocations and major product.
(b)
Interpretation:
The detailed mechanism for the production of given compounds from respective alkyne is to be drawn.
Concept introduction:
The alkynes are electron rich system like alkenes and can undergo an electrophilic addition reaction with strong Bronsted acids just like the alkenes do. The reaction proceeds with proton transfer reaction to form a stable carbocation followed by the action of water as a nucleophile. In excess of reagent, the reaction occurs twice forming a geminal dihalide compound as a major product.
Answer to Problem 11.39P
The detailed mechanism for the given reaction with the major product is:
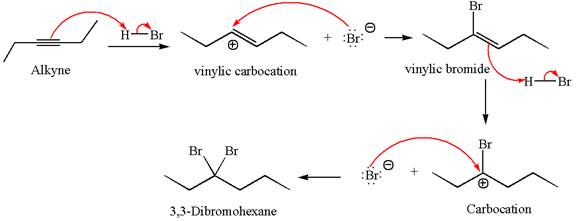
Explanation of Solution
The structure for the given compound
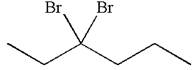
The given compound is germinal dibromide, thus can be produced by electrophilic addition of an excess of
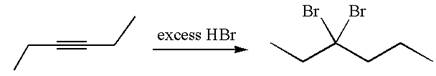
In the given reaction the alkyne is the electron rich site and the hydrogen from the
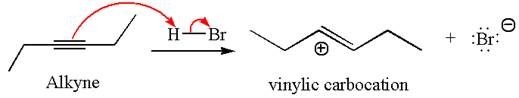
In the second step, the bromide ion acts as a nucleophile and attacks at vinylic carbocation forming vinylic bromide product.
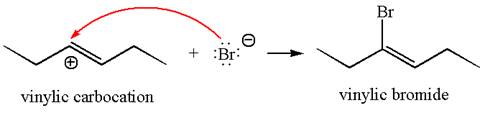
The vinylic bromide again undergoes the addition of
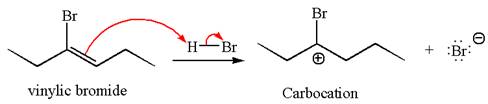
This carbocation further reacts with nucleophilic bromide ion to form germinal dibromide product.
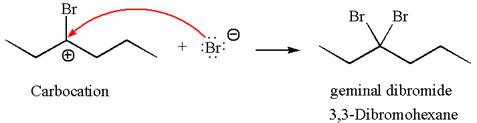
The detailed mechanism is drawn for the given reaction with showing the formation of stable carbocations and major product.
(c)
Interpretation:
The detailed mechanism for the production of given compounds from respective alkyne is to be drawn.
Concept introduction:
The alkynes are electron rich system like alkenes and can undergo an electrophilic addition reaction with strong Bronsted acids just like the alkenes do. The reaction proceeds with proton transfer reaction to form a stable carbocation followed by the action of water as a nucleophile. In a single addition reaction, the reaction occurs only once forming a vibylic halide compound as a major product. The deuterium is an isotope of a hydrogen atom and reacts the same as hydrogen.
Answer to Problem 11.39P
The detailed mechanism for the given reaction with a major product is:
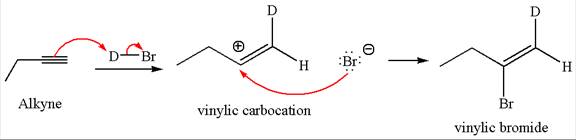
Explanation of Solution
The structure for the given compound is:
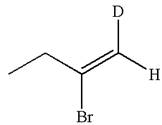
The given compound is vinylic bromide having deuterium at adjacent carbon, thus can be produced by single electrophilic addition of
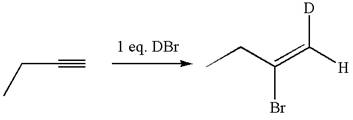
In the given reaction the alkyne is the electron rich site and the hydrogen from the
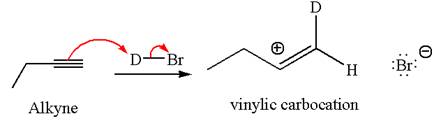
In the second step the bromide ion acts as a nucleophile and attacks at vinylic carbocation forming vinylic bromide product.
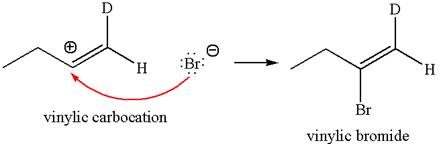
The detailed mechanism is drawn for the given reaction with showing the formation of stable carbocations and major product.
(d)
Interpretation:
The detailed mechanism for the production of given compounds from respective alkyne is to be drawn.
Concept introduction:
The alkynes are electron rich system like alkenes and can undergo an electrophilic addition reaction with strong Bronsted acids just like the alkenes do. The reaction proceeds with proton transfer reaction to form a stable carbocation followed by the action of water as a nucleophile. In excess of reagent, the reaction occurs twice forming a geminal dihalide compound as a major product.
Answer to Problem 11.39P
The detailed mechanism for the given reaction with the major product is:
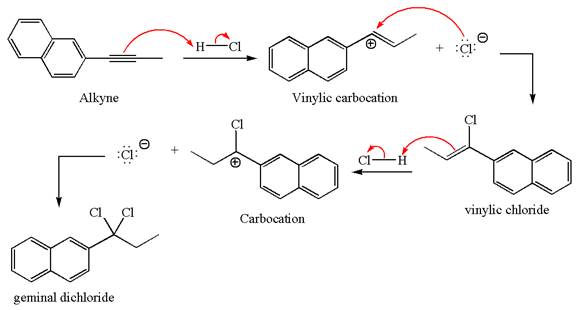
Explanation of Solution
The structure for the given compound is:
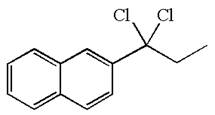
The given compound is germinal dichloride, thus can be produced by electrophilic addition of an excess of
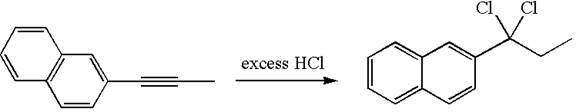
In the given reaction the alkyne is the electron rich site and the hydrogen from the
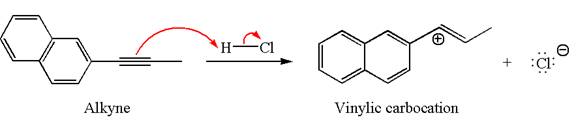
In the second step, the chloride ion acts as a nucleophile and attacks at vinylic carbocation forming vinylic chloride products.
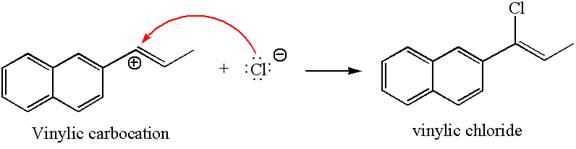
The vinylic chloride again undergoes the addition of
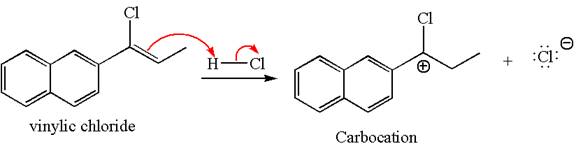
This carbocation further reacts with nucleophilic chloride ion to form a germinal dichloride product.
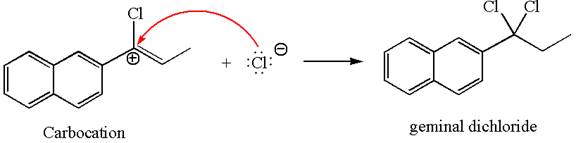
The detailed mechanism is drawn for the given reaction with showing the formation of stable carbocations and major product.
Want to see more full solutions like this?
Chapter 11 Solutions
ORGANIC CHEMISTRY E-BOOK W/SMARTWORK5
- Solve correctly please. What is the mechanism and the product of the following reaction?arrow_forwardProvide the full mechanism and major products for each of the following reactions.arrow_forwardprovide the major product(s) for each of the following reactions. also, provide the mechanism for the last two reactionsarrow_forward
- provide full and detailed reaction mechanism given the following reactants, products and conditionsarrow_forwardPredict the major product of each of the following reactions and provide the complete, detailed mechanismarrow_forwardGiven the detailed reaction mechanism that predicts the terminal alkyne product of this reaction.arrow_forward
- Given the information below, write out a reasonable mechanism for the reaction. Where not provided, also fill in the major product(s) of the reaction: Show all arrowsarrow_forwardPlease predict the product of the following reaction, and draw the complete, detailed mechanism.arrow_forwardProvide mechanism of this reactionarrow_forward
- Provide mechanism for the following reactionarrow_forwardany plausible reasoning how this could occur, please show mechanism.arrow_forward(SYN) Show how to synthesize the following molecule from any compounds containing two carbons. Draw the complete, detailed mechanism for the reaction.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





