
Concept explainers
Benzocaine is the active ingredient in topical pain relievers such as Orajel and Anbesol. Give the molecular formula for benzocaine.
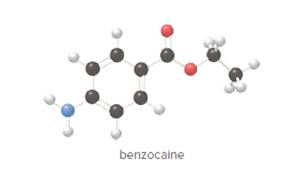
- Give the molecular formula for benzocaine.
- Draw in all lone pairs on heteroatoms using a skeletal structure.
- How many trigonal planar carbons does benzocaine contain?
- Predict the water solubility of benzocaine.
- Label all polar bonds.
(a)
Interpretation:
To determine the molecular formula of benzocaine from the ball and stick model.
Concept Introduction:
In ball and stick model, black color ball represents carbon atom, white color ball represents hydrogen atom, red color ball represents oxygen atom and blue color ball represents nitrogen atom. To determine the molecular formula, convert the ball and stick model to complete structure.
Answer to Problem 81P
Molecular formula of benzocaine is
Explanation of Solution
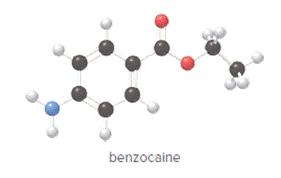
The compound is as follows:
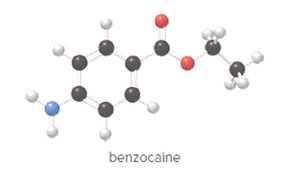
Convert ball and stick model to normal structure. Replace black balls by carbon atoms, red balls by oxygen atoms, blue ball by nitrogen atom and whit balls by hydrogen atom. But, all the bonds between atoms are same.
In benzocaine, nine carbon atoms, eleven hydrogen atoms, two oxygen atoms and one nitrogen atom present. Hence, molecular formula of benzocaine is
(b)
Interpretation:
To draw all lone pairs on heteroatoms of benzocaine using a skeletal structure.
Concept Introduction:
Heteroatoms are those atoms in an organic compound other than carbon and hydrogen atom like oxygen, nitrogen, etc. Lone pairs are pair of electrons available on an atom after bond formation.
Answer to Problem 81P
The structure of benzocaine having all lone pairs on heteroatoms is as follows:
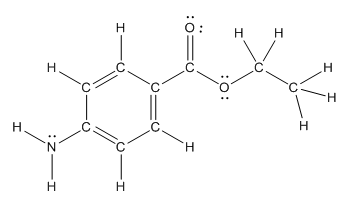
Explanation of Solution
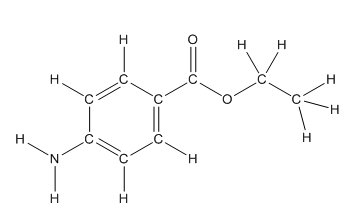
Skeletal structure of benzocaine is as follows:
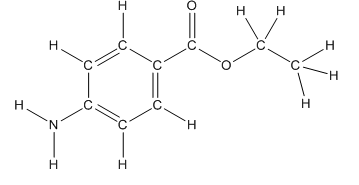
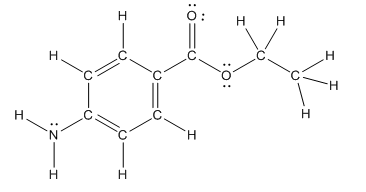
The heteroatoms present in benzocaine are nitrogen atom and two oxygen atoms. Nitrogen has five valence electrons. Out of five electrons, two electrons are used for making two (N-H) bonds, one electron is used for making one N-C bond. Remaining two electrons forms a lone pair. Oxygen has six valence electrons. For double bonded oxygen atom, two electrons are used for making the double bond. Remaining four electrons forms two lone pair. Other oxygen atom forms two bonds with carbon atoms means two electrons are used in bond formation. So, two pairs of lone pair present. Hence, the structure having all lone pairs on heteroatoms is as follows:
(c)
Interpretation:
To determine the number of carbon atoms having trigonal planar shape in benzocaine.
Concept Introduction:
The following table should be used while determining shape around an atom.
| Number of groups | Number of atoms | Number of lone pairs | Shape | Bond angle |
| 2 | 2 | 0 | Linear | |
| 3 | 3 | 0 | Trigonal planar | |
| 4 | 4 | 0 | Tetrahedral | |
| 4 | 3 | 1 | Trigonal pyramidal | |
| 4 | 2 | 2 | Bent |
If an atom is surrounded by three groups, then the shape around that particular atom is trigonal planar.
Answer to Problem 81P
In benzocaine, seven carbon atoms have trigonal planar structure.
Explanation of Solution
If an atom is surrounded by three groups, then the shape around that particular atom is trigonal planar.
Structure of benzocaine is as follows:
To find the number of carbon atoms having trigonal planar shape in benzocaine, observe the carbon atoms and find which carbon atom has three groups surround it.
All the carbon atoms in the benzene ring have three atoms around them also the carbon bonded to the benzene ring has three groups surround it. So, seven carbon atoms in benzocaine have trigonal planar structure.
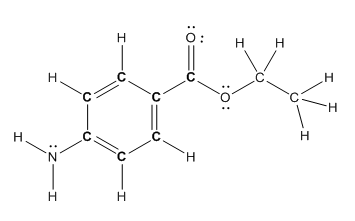
The bold carbon atoms have trigonal planar structure.
(d)
Interpretation:
To predict the solubility of benzocaine in water.
Concept Introduction:
Water is a polar solvent. To dissolve in water the compound must be polar. Polar compound is that compound in which polar bonds are present. The unequal sharing of valence electrons in a bond is called polar bond. Polar bond result when the bond formed between two atoms in which one atom is more electronegative than the other one. One example of polar bond is
Structure of HCl is as follows:

In
Answer to Problem 81P
Benzocaine is water soluble compound.
Explanation of Solution
The structure of benzocaine is as follows:
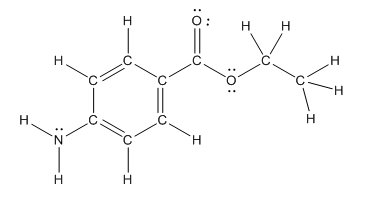
As the compound contains heteroatoms like nitrogen, oxygen the compound is polar that is, they can form hydrogen bonds with water. So, benzocaine is water soluble compound.
(e)
Interpretation:
To label all the polar bonds in benzocaine.
Concept Introduction:
The unequal sharing of valence electrons in a bond is called polar bond. Polar bond result when the bond formed between two atoms in which one atom is more electronegative than the other one. One example of polar bond is
Structure of HCl is as follows:

In
Answer to Problem 81P
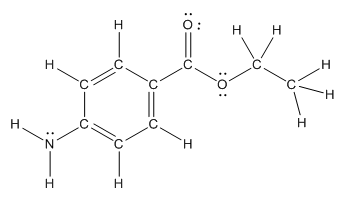
Polar bond in benzocaine are as follows:
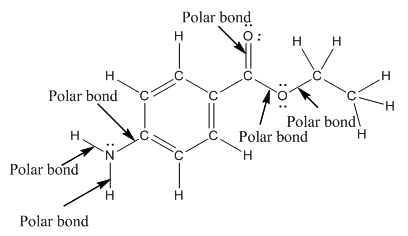
Explanation of Solution
The structure of benzocaine is as follows:
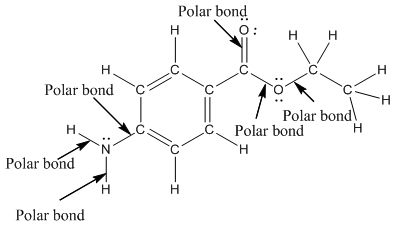
In organic compound, most of the polar bonds formed between carbon and heteroatoms like oxygen, nitrogen, sulphur etc. In benzocaine six polar bonds present that is, three polar bond between carbon and oxygen (oxygen is more electronegative than carbon), two polar bond between nitrogen and hydrogen (nitrogen is more electronegative than hydrogen) and on polar bond between carbon and nitrogen (nitrogen is more electronegative than carbon).
Want to see more full solutions like this?
Chapter 11 Solutions
General, Organic, and Biological Chemistry - 4th edition
- Chloroform CHCl3 is your compound saturated or unsaturated? Do any geometric isomers exist in point format what functional groups does your chemical contain in point format And add any other information that you learned about chloroform in point formatarrow_forwardPlease help for all questions A) Convert the structure in condensed form.b) Identify one the functional group with its respective homologous series that exists in thecompound.c) Draw one possible structure with molecular formula C6H12 that has two tertiary carbon.d) Arrange the following compounds in increasing order of melting point.Hexane 3-methylhexane 3-ethylhexane 2,3-dimethylpentanearrow_forwardIdentify and label all functional groups (alcohols, amines, amides, carboxylic acids, ketones, aldehydes, aromatic rings, aromatic amines, etc) of Carvone on the Lewis Structure. You do not have to circle alkanes. Make sure to add additional details about the functional groups of Carvonearrow_forward
- How does the structure of an alcohol differ from an ether? Describe how an aldehyde differs in structure from a ketone. Thiols are compounds which resemble alcohols, except that the oxygen atom is replaced by a sulfur atom. Draw the analogous thiol for the four carbon alcohol in Table 1. Describe the structural difference between carboxylic acids and esters. Are ethers polar molecules? Would you expect ethers to have higher or lower boiling points than alkanes (circle one)? Explain. Pentane (an alkane) has a boiling point of 36 °C. Does the data agree with your prediction? explain why this could be the casearrow_forwardAnswer the following questions by referring to the ball-and-stick model of fentanyl, a potent narcotic analgesic used in surgical procedures. a. Identify the functional groups. b. Label the most acidic proton. c. Label the most basic atom. d. What types of intermolecular forces are present between two molecules of fentanyl? e. Draw an isomer predicted to have a higher boiling point. f. Which sites in the molecule can hydrogen bond to water? g. Label all electrophilic carbons.arrow_forwardAnswer the following questions by referring to the ball-and-stick model of fentanyl, a potent narcotic analgesic used in surgical procedures.a.Identify the functional groups. b. Label the most acidic proton. c.Label the most basic atom. d.What types of intermolecular forces are present between two molecules of fentanyl? e.Draw an isomer predicted to have a higher boiling point. f.Which sites in the molecule can hydrogen bond to water? g.Label all electrophilic carbons.arrow_forward
- Draw structures that fit each description and name the functional group in each molecule: (a) two constitutional isomers with molecular formula C5H10O that contain different functional groups; (b) two constitutional isomers with molecular formula C6H10O that contain the same functional group.arrow_forwardUnlike methanol, which is a nearly odorless liquid, methanethiol (CH3SH) is a gas with an appalling odor reminiscent of skunks. It is one of the compounds added to natural gas as a warning for gas leaks. Using appropriate drawing tools, draw the structure of methanethiol in such a way that there is double bond between carbon and sulfur.arrow_forwardEncircle and identify all functional groups in each compound.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning



