
Concept explainers
(a)
Interpretation:
The shape around the labelled atoms needs to be determined.
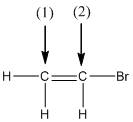
Concept Introduction:
Shape of a molecule is determined by including only the bond pair not lone pairs on the central atom while geometry includes both the bond pairs and lone pairs on the central atom. Valence shell electron pair repulsion theory or VSEPR theory used in chemistry as a model for the prediction of shape of various molecules by knowing the electron pairs on the central atom. There will be repulsion between the electron pairs present on central atom, so to minimize the repulsion they adopt an arrangement with minimum repulsion, thus determining molecule's shape. And by knowing the shape we can easily determine the bond angles.
The following table should be used while determining the shapes:
| Number of groups | Number of lone pairs | Shape | Bond angle | |
| 2 | 2 | 0 | Linear | |
| 3 | 3 | 0 | Trigonal planar | |
| 4 | 4 | 0 | Tetrahedral | |
| 4 | 3 | 1 | Trigonal pyramidal | |
| 4 | 2 | 2 | Bent |
(b)
Interpretation:
The shape around the labelled atoms needs to be determined.
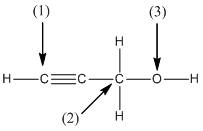
Concept Introduction:
Shape of a molecule is determined by including only the bond pair not lone pairs on the central atom while geometry includes both the bond pairs and lone pairs on the central atom. Valence shell electron pair repulsion theory or VSEPR theory used in chemistry as a model for the prediction of shape of various molecules by knowing the electron pairs on the central atom. There will be repulsion between the electron pairs present on central atom, so to minimize the repulsion they adopt an arrangement with minimum repulsion, thus determining molecule's shape. And by knowing the shape we can easily determine the bond angles.
The following table should be used while determining the shapes:
| Number of groups | Number of atoms | Number of lone pairs | Shape | Bond angle |
| 2 | 2 | 0 | Linear | |
| 3 | 3 | 0 | Trigonal planar | |
| 4 | 4 | 0 | Tetrahedral | |
| 4 | 3 | 1 | Trigonal pyramidal | |
| 4 | 2 | 2 | Bent |
(c)
Interpretation:
The shape around the labelled atoms needs to be determined.
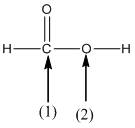
Concept Introduction:
Shape of a molecule is determined by including only the bond pair not lone pairs on the central atom while geometry includes both the bond pairs and lone pairs on the central atom. Valence shell electron pair repulsion theory or VSEPR theory used in chemistry as a model for the prediction of shape of various molecules by knowing the electron pairs on the central atom. There will be repulsion between the electron pairs present on central atom, so to minimize the repulsion they adopt an arrangement with minimum repulsion, thus determining molecule's shape. And by knowing the shape we can easily determine the bond angles.
The following table should be used while determining the shapes:
| Number of groups | Number of atoms | Number of lone pairs | Shape | Bond angle |
| 2 | 2 | 0 | Linear | |
| 3 | 3 | 0 | Trigonal planar | |
| 4 | 4 | 0 | Tetrahedral | |
| 4 | 3 | 1 | Trigonal pyramidal | |
| 4 | 2 | 2 | Bent |
(d)
Interpretation:
The shape around the labelled atoms needs to be determined.
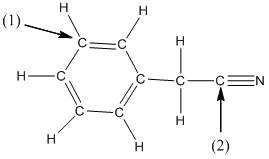
Concept Introduction:
Shape of a molecule is determined by including only the bond pair not lone pairs on the central atom while geometry includes both the bond pairs and lone pairs on the central atom. Valence shell electron pair repulsion theory or VSEPR theory used in chemistry as a model for the prediction of shape of various molecules by knowing the electron pairs on the central atom. There will be repulsion between the electron pairs present on central atom, so to minimize the repulsion they adopt an arrangement with minimum repulsion, thus determining molecule's shape. And by knowing the shape we can easily determine the bond angles.
The following table should be used while determining the shapes:
| Number of groups | Number of atoms | Number of lone pairs | Shape | Bond angle |
| 2 | 2 | 0 | Linear | |
| 3 | 3 | 0 | Trigonal planar | |
| 4 | 4 | 0 | Tetrahedral | |
| 4 | 3 | 1 | Trigonal pyramidal | |
| 4 | 2 | 2 | Bent |
Want to see the full answer?
Check out a sample textbook solution
Chapter 11 Solutions
General, Organic, and Biological Chemistry - 4th edition
- Name each compound: NO, NO2, SO2, H2SO4, HNO3, CaCO3arrow_forwardHow many hydrogen atoms are present around each highlighted carbon atom in the following molecules? What is themolecular formula for each molecule? Both compounds are active ingredients in some common sunscreens.arrow_forwardDetermine the number of lone pairs on each oxygen, nitrogen, and charged carbon atoms in the following structures.arrow_forward
- What is the functional group in each compound?arrow_forwardHow many electron pairs are shared when a triple bond exists between two carbon atoms? What must he the geometric arrangement around the carbon atoms in a triple bond? Draw the Lewis structure of a simple molecule that contains a triple bond.arrow_forward
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co





