
Concept explainers
(a)
Interpretation:
Whether the following molecule is polar or non-polar should be determined:
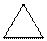
Concept introduction:
Polarity of any individual bond is determined by the electro-negativities of the atoms forming a bond. Any covalent bond is non-polar when two identical atoms or of similar electro-negativity are bonded together.
(b)
Interpretation:
Whether the following molecule is polar or non-polar should be determined:
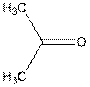
Concept introduction:
Polarity of any individual bond is determined by the electro-negativities of the atoms forming a bond. Any covalent bond is non-polar when two identical atoms or of similar electro-negativity are bonded together.
(c)
Interpretation:
Whether
Concept introduction:
Polarity of any individual bond is determined by the electro-negativities of the atoms forming a bond. Any covalent bond is non-polar when two identical atoms or of similar electro-negativity are bonded together.
(d)
Interpretation:
Whether
Concept introduction:
Polarity of any individual bond is determined by the electro-negativities of the atoms forming a bond. Any covalent bond is non-polar when two identical atoms or of similar electro-negativity are bonded together.
Want to see the full answer?
Check out a sample textbook solution
Chapter 11 Solutions
General, Organic, and Biological Chemistry - 4th edition
- Which of the following molecules is(are) polar? For each polar molecule indicate the direction of polaritythat is, which is the negative end, and which is the positive end of the molecule. (a) BeCl2 (b) HBF2 (c) CH3Cl (d) SO3arrow_forwardIs the Cl2BBCl2 molecule polar or nonpolar?arrow_forwardDetermine whether each molecule is polar or nonpolar: CF4, CH4, NH3. EN values: C: 2.6; H: 2.2; F: 4.0; N: 3.0.Start with Lewis structure then draw the shape of the molecule →determine the polarity of the bond(s) →provide the orientation of the dipoles to determine polarity of the moleculearrow_forward
- Arrange the molecules according to decreasing molecular polarity. A. I, II, III, IV B. IV, III, II, I C. II, IV, I, III D. III, I, IV, IIarrow_forwarda) how many total pairs of electrons does CF4 have? b) How many bonded pairs and how many lone pairs does CF4 have? c) What is the molecular shape and angle of CF4? d) what is the lewis structure for CF4?arrow_forwardIdentify the electron pair geometry, the molecular structure of each, and the polarity of the following molecules: (a) ClNO (N is the central atom) (b) Cl2CO (C is the central atom) (c) Cl2SO (S is the central atom) (d) SO2F2 (S is the central atom) (e) XeO2F2 (Xe is the central atom) (f) ClOF2 + (Cl is the central atom)arrow_forward
- Which of the following molecules contain polar covalent bonds ONLY? I. CH4 II. KNO3 III. H2O a. I, II, and III b. III ONLY c. I ONLY d. II ONLY e. II AND III ONLY f. I AND III ONLYarrow_forwardClassify each as ionic, covelent non polar or covelent polar C-S Na-Br N-N C-Carrow_forwardMatch each compound with one of the molecular shapes shown below ( C or D): (a) BBr 3; (b) NBr 3; (c) PBr 3.arrow_forward
- In the following list, which one of the substances below contains POLAR COVALENT bonds. A H2O B LiF C CaCl2 D H2arrow_forwardLabel the polar bonds in each molecule, and then decide if the molecule is polar or nonpolar. a.) HCl b.) C2H6 c) CH2F2 d.) HCN e.) CCl4arrow_forwardYou come across a bottle in the stock room labeled CHCl3 a.Draw the Lewis dot structure of the molecule b.Identify the molecular geometry of the molecule c.dentify if the molecule is polar or non-polar. d.Do you expect this molecule to interact more with water (H2O) or methane (CH4)?Explain why.arrow_forward
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning





