
a)
Interpretation:
The expected product between the
Answer to Problem 21VC
The ethyl chloride undergoes SN2 reaction with Na+SCH3- and NaOH to yield a product.
The product is CH3CH2SCH3, CH3CH2OH.
Here the leaving group is Cl.
The nucleophile is SCH3-,OH.
Hence it undergoes SN2 reaction.
Explanation of Solution
The ethyl chloride undergoes SN2 reaction with Na+SCH3- and NaOH to yield a product.
The product is CH3CH2SCH3, CH3CH2OH.
Here the leaving group is Cl.
The nucleophile is SCH3-,OH.
Hence it undergoes SN2 reaction.
The ethyl chloride undergoes SN2 reaction with Na+SCH3- and NaOH to yield a product.
The product is CH3CH2SCH3, CH3CH2OH.
Here the leaving group is Cl.
The nucleophile is SCH3-,OH.
Hence it undergoes SN2 reaction.
b)
Interpretation:
The expected product between the alkyl halide CH3CH2Cl+Na+SCH3- and NaOH is interpreted.
Answer to Problem 21VC
The alkyl halide undergoes SN1 reaction with Na+SCH3- to yield a SCH3 Substituited product.
The product is shown in the reaction.
In this reaction leaving group is Cl, the nucleophile is SCH3-
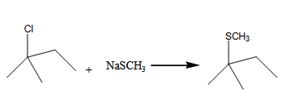
Explanation of Solution
The alkyl halide undergoes E1 reaction with NaOH to yield a
The product is shown here
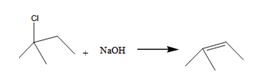
The alkyl halide undergoes SN1reaction with Na+SCH3- to yield a SCH3 Substituited product.
The product is shown in the reaction.
In this reaction leaving group is Cl, the nucleophile is SCH3-

c)
Interpretation:
The expected product between the alkyl halide CH3CH2Cl+Na+SCH3- and NaOH is interpreted.
Answer to Problem 21VC
The benzylchloride undergoes SN1 reaction with Na+SCH3- to yield a product.
The product is shown here.
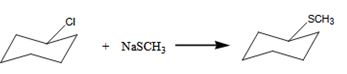
Here the leaving group is Cl.
The nucleophile is SCH3-Hence it undergoes SN1 reaction.
Explanation of Solution
The benzylchloride undergoes E1 reaction with NaOH to yield a product.
The product is shown here
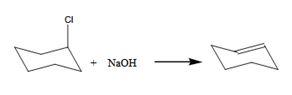
The benzylchloride undergoes SN1 reaction with Na+SCH3- to yield a product.
The product is shown here.
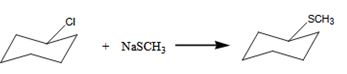
Here the leaving group is Cl.
The nucleophile is SCH3- Hence it undergoes SN1 reaction.
Want to see more full solutions like this?
Chapter 11 Solutions
ORGANIC CHEM.(LL)-W/OWL V2 >CUSTOM<
- Treatment of 1-methylcyclohexene with H3O+ would be expected to yield as the major product which of the molecules below? I, III and V II III and V IV I and IIIarrow_forwardProvide the product most likely to form from reaction of each reagent with 1-methylcyclohexene. You may ingore stereochemistry for this question. Br2 H-Br H-Br, PhC(O)OOC(O)Ph N-bromosuccinimide, PhC(O)OOC(O)Ph A. B. C. D.arrow_forwardReaction of HBr with 2-methylpropene yields 2-bromopropane. What is the structure of the carbocation intermediate formed during the reaction? Show the mechanism of the reaction.arrow_forward
- Show how free-radical halogenation might be used to synthesize the followingcompounds. In each case, explain why we expect to get a single major product.(a) 1-chloro-2,2-dimethylpropane (neopentyl chloride)(b) 2-bromo-2-methylbutanearrow_forwardWhat products would you expect from reaction of the following alkenes with NBS? If more than one product is formed, show the structures of all.arrow_forwardDraw structural formulas for the major product(s) formed by reaction of 3-hexyne with each of these reagents. (Where you predict no reaction, write NR.) Q.) Na in NH3(l )arrow_forward
- Predict the products of the following reactions. ) 1-bromo-4-fluorocyclohexane + MgTHFTarrow_forwardPredict the major products of the following reactions.a.4@chlorocycloheptene + Hg(OAc)2 in CH3OH b.the product from part (c), treated with NaBH4arrow_forwardPredict dehydrohalogenation products that results when these alkyl halides are heated in alcoholic KOH. With more than 1 product, predicting the major and minor productsarrow_forward
- Predict the major products of the following reactions.(a) 1@methylcyclohexene + aqueous Hg(OAc)2 (b) the product from part (a), treated with NaBH4arrow_forwardShow how the dehalogenation of (1R,2R)-1,2-dibromo-1,2-diphenyl ethane with zinc affords cis-stilbene. Show the mechanism with pictures.arrow_forwardWhat would the major organic reaction product be from the reaction of 1-bromo-1-methylcyclopentane withsodium hydroxide? Would the elimination reaction outcome be affected if a student accidentally adds sodium tertbutoxide instead of sodium hydroxide?arrow_forward
