
(a)
Interpretation:
It is to be explained whether the given transformation would be a result of acid-catalyzed hydration or oxymercuration-reduction.
Concept introduction:
The acid-catalyzed hydration of an
The oxymercuration-reduction is also the reaction of addition of water through the
Answer to Problem 12.44P
The given transformation can be carried out by oxymercuration-reduction. The detailed mechanism is as follows:
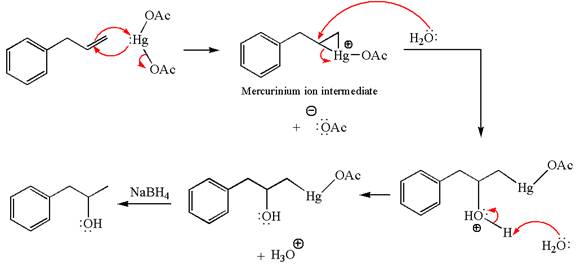
Explanation of Solution
The given equation is
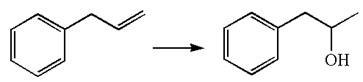
In the substrate, the alkene
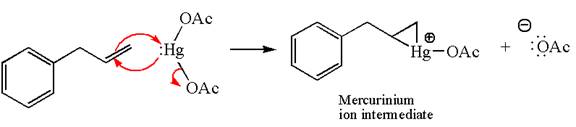
The alkene substrate, on reaction with mercury
In the first step, the electron rich
In the second step, the water molecule acts as a nucleophile on one of the carbons of the three-membered ring to open the ring, followed by deprotonation of the positively charged oxygen atom.
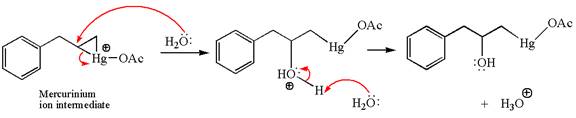
The product formed in the previous step is then subjected to reduction with sodium borohydride,
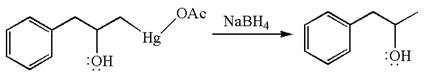
The preparation of the given compound is explained indicating the addition of water across the
(b)
Interpretation:
It is to be explained whether the given transformation would be a result of acid-catalyzed hydration or oxymercuration-reduction.
Concept introduction:
The acid-catalyzed hydration of an alkene is the electrophilic addition of water across the
The oxymercuration-reduction is also the reaction of addition of water through the
Answer to Problem 12.44P
The given transformation can be carried out by acid-catalyzed hydration. The detailed mechanism is as follows:
Explanation of Solution
The given equation is
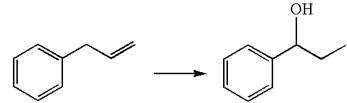
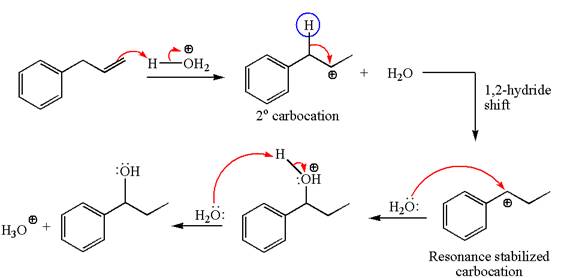
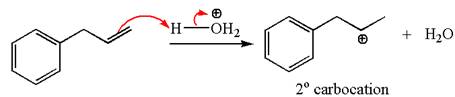
In the substrate, the alkene
The first step is the formation of a secondary carbocation by proton transfer reaction. The proton transfers to the less substituted double bonded carbon.
The secondary carbocation can be rearranged to more stable tertiary as well as resonance stabilized carbocation by
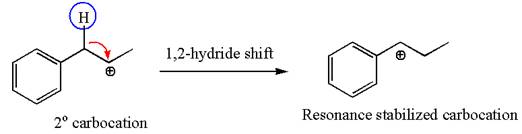
In the second step, the water molecule acts as a nucleophile on one of the carbons of the three-membered ring to open the ring, followed by deprotonation of the positively charged oxygen atom.
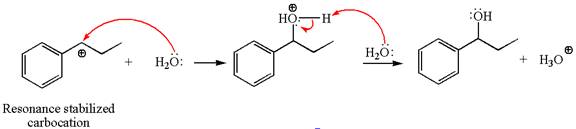
The detailed mechanism for the given reaction is drawn by suggesting that the reaction occurred through carbocation rearrangement.
(c)
Interpretation:
It is to be explained whether the given transformation would be a result of acid-catalyzed hydration or oxymercuration-reduction.
Concept introduction:
The acid-catalyzed hydration of an alkene is the electrophilic addition of water across the
The oxymercuration-reduction is also the reaction of addition of water through the
Answer to Problem 12.44P
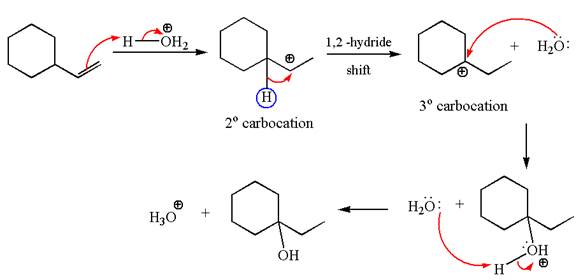
The given transformation can be carried out by acid catalyzed hydration. The detailed mechanism is as follows:
Explanation of Solution
The given equation is
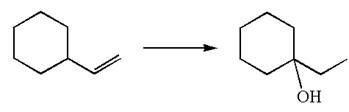
In the substrate, the alkene
The first step is the formation of a secondary carbocation by proton transfer reaction. The proton transfers to the less substituted double bonded carbon.
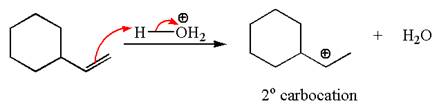
The secondary carbocation can be rearranged to more stable tertiary by
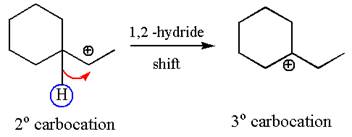
In the second step, the water molecule acts as a nucleophile on one of the carbons of the three-membered ring to open the ring, followed by deprotonation of the positively charged oxygen atom.
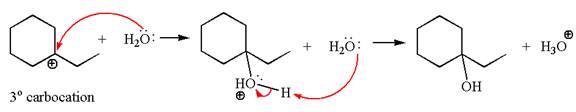
The detailed mechanism for the given reaction is drawn by suggesting that the reaction occurred through carbocation rearrangement.
(d)
Interpretation:
It is to be explained whether the given transformation would be a result of acid-catalyzed hydration or oxymercuration-reduction.
Concept introduction:
The acid-catalyzed hydration of an alkene is the electrophilic addition of water across the
The oxymercuration-reduction is also the reaction of addition of water across the
Answer to Problem 12.44P
The given transformation can be carried out by oxymercuration-reduction. The detailed mechanism is as follows:
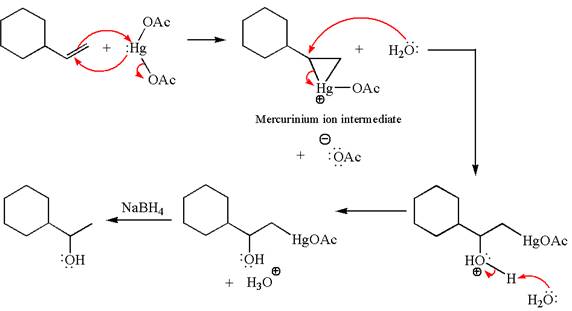
Explanation of Solution
The given equation is
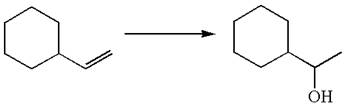
In the substrate, the alkene
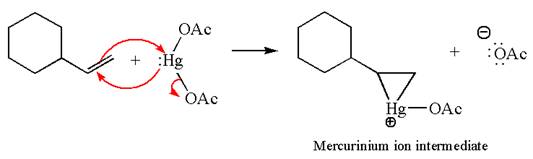
The alkene substrate, on reaction with mercury
In the first step, the electron rich
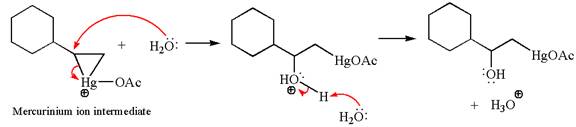
In the second step, the water molecule acts as a nucleophile on one of the carbons of the three-membered ring to open the ring, followed by deprotonation of the positively charged oxygen atom.
The product formed in the previous step is then subjected to reduction with sodium borohydride,
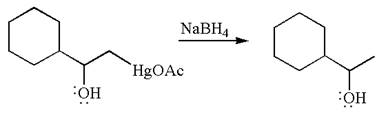
The preparation of the given compound is explained indicating the addition of water across the
Want to see more full solutions like this?
Chapter 12 Solutions
ORGANIC CHEMISTRY E-BOOK W/SMARTWORK5
- Which of these alkenes will produce a more stable intermediate via 1,2-hydride shift when it is reacted to undergo acid catalyzed hydration?arrow_forwardMulti-step synthesis. Give the reagents over the arrows and the intermediates between thearrows that are required to synthesize the product compound on the left from the reactant givenon the right for each retrosynthetic analysis.arrow_forwardGive the major organic product(s) for a step of the following sequence of reaction. Write NR if the reaction will not occur.arrow_forward
- Which of the following will not undergo catalytic reduction in the presence of H2 and metal catalyst?arrow_forwardThe following molecule undergoes an intramolecular reaction in the presence of pyrrolidinium acetate, the protonated form of pyrrolidine. Draw the product of this reaction, assuming that a dehydration reaction takes place.arrow_forwardwrite the complete reaction mechanism for the formation of compound B and compound C in this reactionarrow_forward
- Identify reagents and catalyst used or major product obtained in each of the following reactions:arrow_forwardProvide the organic product for the following reaction:arrow_forwardWhat is the final product of the following reaction sequence? Give structural details of all significant intermediates.arrow_forward
- The reaction that occurs when the benzaldehyde you have is reacted in a basic environment is called the Cannizzaro reaction, and when it is reacted with cyanide, it is called benzoin. It Explain the reason by writing the reaction mechanisms of the reactions.arrow_forwardIn the double elimination reaction involving the synthesis of diphenylacetylene from meso 1,2-dibromo-1,2-diphenylethane shown, which of the two steps is rate determining and why?arrow_forwardFor the following reaction scheme, identify by drawing the reagents b and d and the intermediate c that are formed in the synthesis of benzoic acid.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
