
(a)
Interpretation:
The mechanism and major organic product(s) of the given reaction are to be drawn.
Concept introduction:
In the hydroboration reaction of an alkene, a molecule of water adds to the carbon-carbon double bond of the alkene in an anti-Markovnikov manner. The boron atom in borane is less electronegative than hydrogen and acts as the electron-poor atom. Therefore, the
Answer to Problem 12.52P
The mechanism for the given reaction is
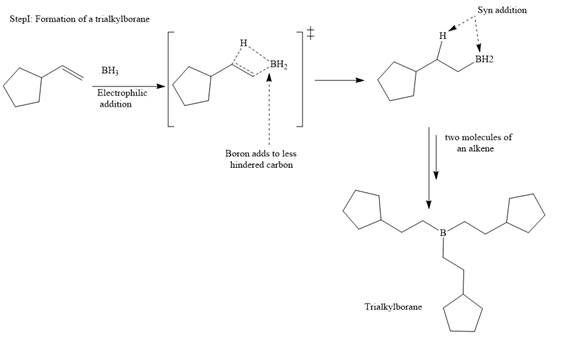
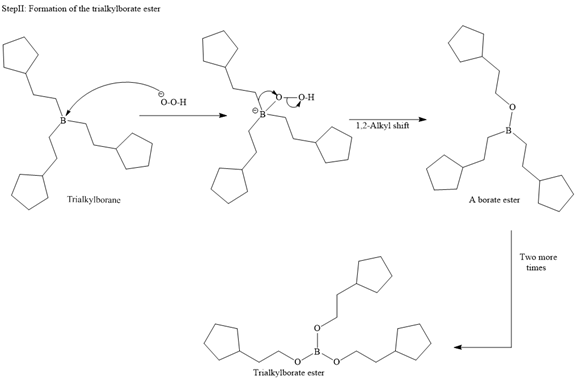
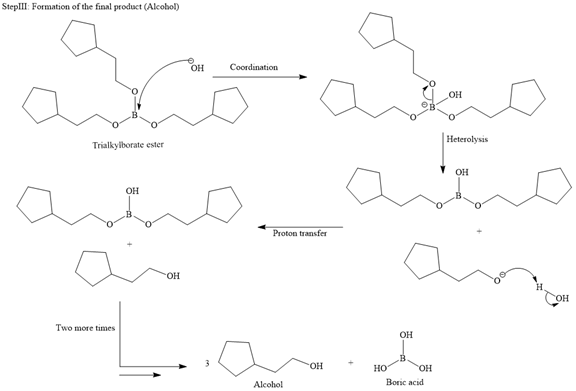
The major product for the given reaction is
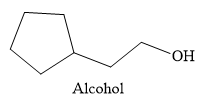
Explanation of Solution
The given reaction is
The structure of cyclopentylethene is
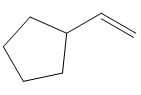
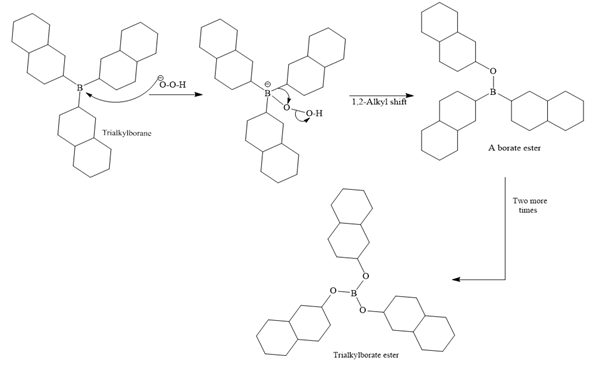
In the first step, borane adds across the carbon-carbon double bond of the alkene via a four-membered cyclic transition state. The addition is anti-Markovnikov as the boron atom adds to the fewer substituted (fewer sterically hindered) carbon, and the hydrogen adds to the most substituted carbon. The remaining two hydrogen atoms from borane are replaced in a similar way by two more alkene molecules to form a trialkylborane.
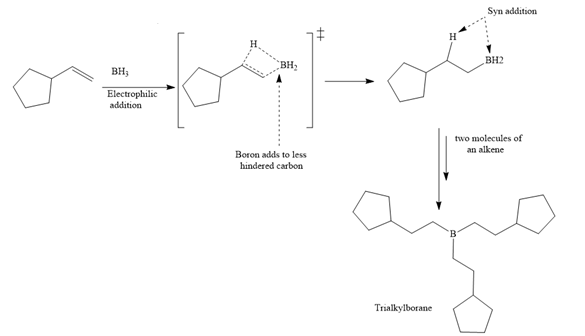
A trialkylborane is then oxidized by the hydroperoxide anion (
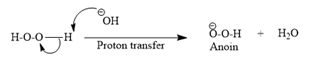
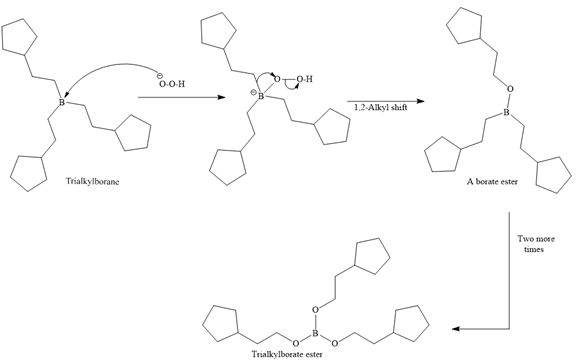
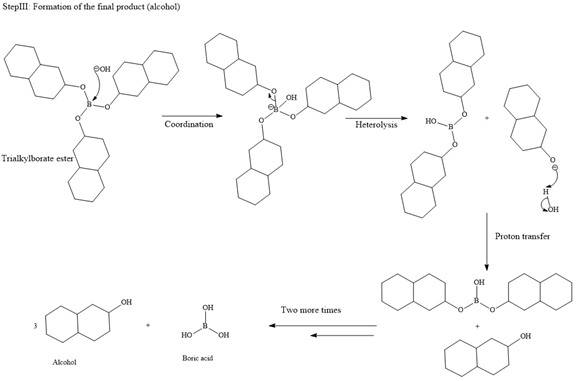
Then the trialkylborate ester undergoes base hydrolysis to three molecules of the alcohol product and boric acid.
The
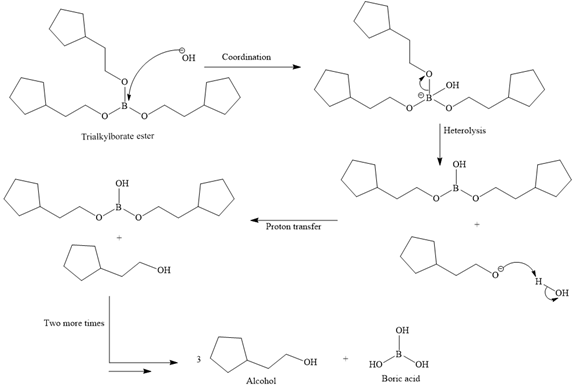
Thus, the major product of the given reaction is

The mechanism and the major organic product(s) for the given reaction are drawn on the basis of an anti-Markovnikov syn addition of borane across the double bond.
(b)
Interpretation:
The mechanism and the major organic product(s) of the given reaction are to be drawn.
Concept introduction:
In the hydroboration reaction of an alkene, a molecule of water adds to the carbon-carbon double bond of the alkene in an anti-Markovnikov manner. The boron atom in borane is less electronegative than hydrogen and acts as the electron-poor atom. Therefore, the
Answer to Problem 12.52P
The mechanism for the given reaction is
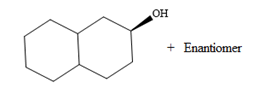
The major product of the given reaction is
Explanation of Solution
The given reaction is
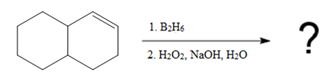
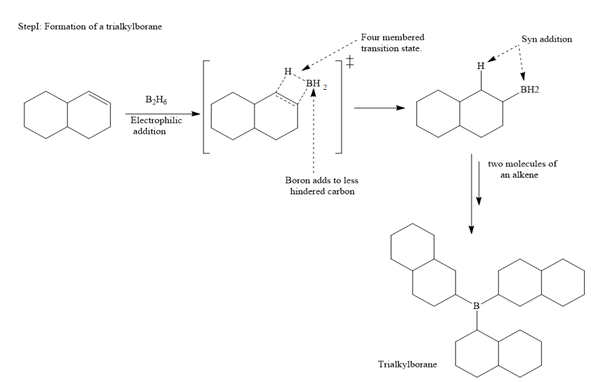
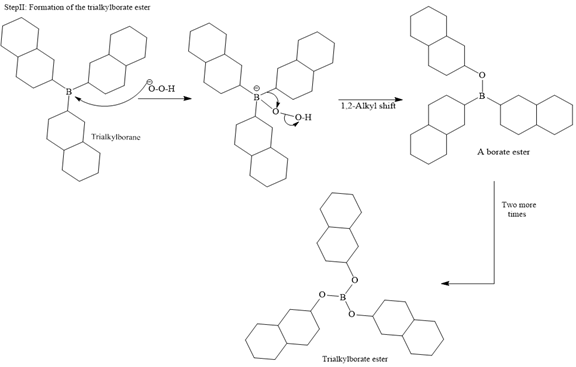
In the first step, borane adds across the carbon-carbon double bond of the alkene via a four-membered cyclic transition state. The addition is anti-Markovnikov as the boron atom adds to the fewer substituted (fewer sterically hindered) carbon, and the hydrogen adds to the most substituted carbon. The remaining two hydrogen atoms from borane are replaced in a similar way by two more alkene molecules to form a trialkylborane.
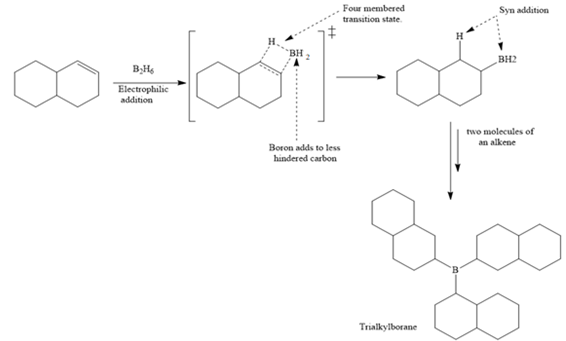
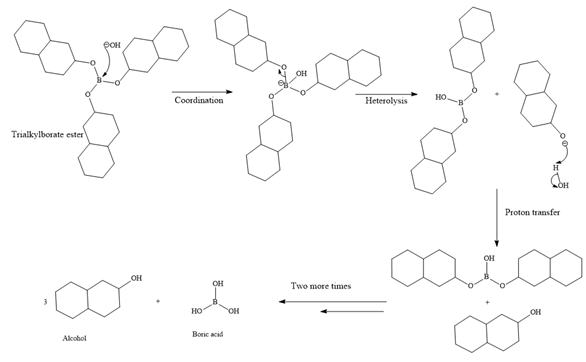
The trialkylborane is then oxidized by the hydroperoxide anion (
Then the trialkylborate ester undergoes base hydrolysis to three molecules of the alcohol product and boric acid.
The
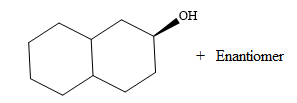
Borane can add across the double bond from either above the plane of the ring or from below the plane of the ring. This will lead to the formation of a racemic mixture as the carbon bonded to the OH group is chiral.
Thus, the major product for the given reaction is
The mechanism and the major organic product(s) for the given reaction are drawn on the basis of an anti-Markovnikov syn addition of borane across the double bond.
(c)
Interpretation:
The mechanism and the major organic product(s) of the given reaction are to be drawn.
Concept introduction:
Like alkenes,
The addition of one molecule of borane produces an alkene, and if it is a terminal alkene, another molecule of borane can add to it. This leads to a mixture of products.
This problem can be avoided by the use of a bulky dialkylborane like disiamylborane,
Answer to Problem 12.52P
The mechanism for the given reaction is
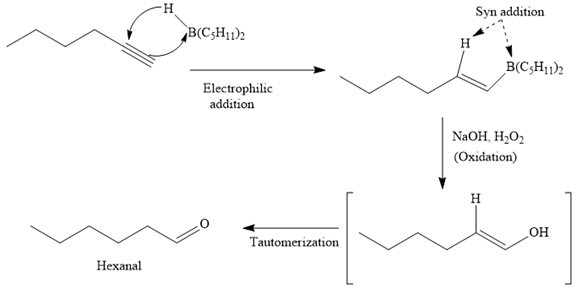
The major product for the given reaction is
Explanation of Solution
The given reaction is
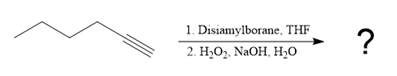
The structure of disiamylborane is
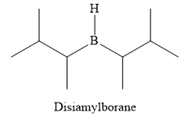
It is a sterically hindered alkyl borane, having only one
So the first step of the above reaction is a syn anti-Markovnikov electrophilic addition of boron and hydrogen to the carbon-carbon triple bond of the substrate.
Oxidation of this adduct by basic

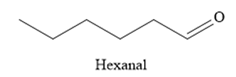
Thus, the major product of the given reaction is
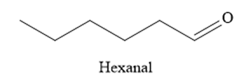
The mechanism and the major organic product(s) of the given reaction are drawn on the basis of the reaction conditions.
(d)
Interpretation:
The mechanism and the major organic product(s) of the given reaction are to be drawn.
Concept introduction:
Like alkenes, alkynes also undergo hydroboration reaction, but the product is different than alcohol. The oxidation of alkynes is carried out by using bulky dialkylborane like disiamylborane
Answer to Problem 12.52P
The mechanism for the given reaction is
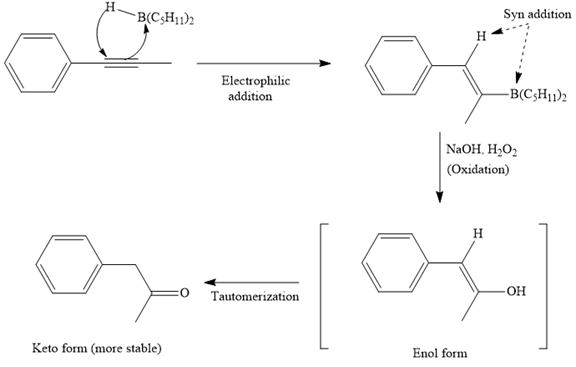
The major product for the given reaction is
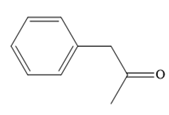
Explanation of Solution
The given reaction is
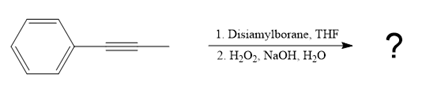
The structure of disiamylborane is
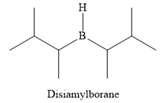
It is a sterically hindered alkyl borane having only one
The first step of the reaction is a syn anti-Markovnikov electrophilic addition of

Thus, the major product for the given reaction is
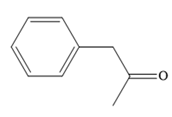
The mechanism and major organic product(s) for the given reaction are drawn on the basis of the reaction conditions.
Want to see more full solutions like this?
Chapter 12 Solutions
ORGANIC CHEMISTRY E-BOOK W/SMARTWORK5
- Draw the mechanism and the major organic product for each of the following reactions. Please show all work with arrowsarrow_forwarddraw all of the mechanism for this given acidarrow_forwardThe reaction shown here is a halosulfonation, which is a useful variation of the sulfonation reaction. Draw the complete mechanism for this reaction.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





