
Concept explainers
Interpretation:
It is to verify that the
Concept introduction:
Bond energy is the measure of bond strength in a chemical bond. In a compound, the nuclei are bind together by
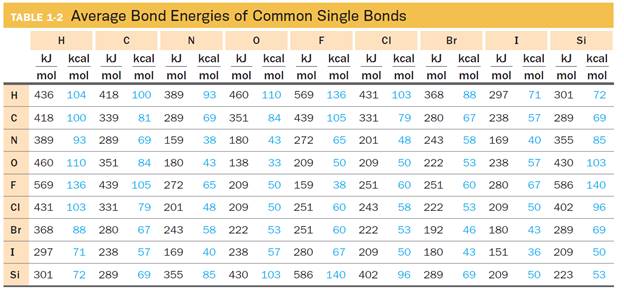
Table 1-2 lists average bond energies for a variety of common bonding partners found in organic species and contains only single bonds:
Want to see the full answer?
Check out a sample textbook solution
Chapter 12 Solutions
ORGANIC CHEMISTRY E-BOOK W/SMARTWORK5
- Consider the following bases: a. For each base above, circle the atom/atoms with the highest PE (will release the most P.E.when a lone pair on this atom combines with an H+ ) b. Rank the bases 1 (highest P.E./strongest base) to 7 (lowest PE/weakest base), and explainyour reasoning.arrow_forwardSecond image is answer that is wrong Please give right answer with explanation please.arrow_forwardANSWER ALL AND SKIP IF YOU ALREADY DID THISarrow_forward
- Kindly answer a, b, carrow_forwardCan you help me with parts f and g? (On second page)arrow_forwardSolve correctly please, with some explanation also. Draw the product(s) for the following radical reaction where one bond breaks and another bond is formed. The curved arrows indicate the movement of an unpaired electron.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
